Steel Service Centers

Steel service centers are companies that specialize in procuring steel directly from mills and manufacturers and supplying them to the customers. They are fundamental to the steel supply chain...
Please fill out the following form to submit a Request for Quote to any of the following companies listed on
This article will provide industry insights into aluminized steel.
Read further to learn:

Aluminized steel refers to steel that is coated with aluminum or aluminum-silicon to significantly enhance its properties, especially its ability to resist corrosion and rust. This protective layer is applied through a hot dipping process, where the steel is immersed in molten aluminum. This not only enhances the surface properties of the steel but also increases its durability and performance, making it comparable to stainless steel.

The production of aluminized steel uniquely combines the resilient structure of steel with aluminum's surface traits, resulting in a material known for its remarkable durability and aesthetic appeal. Steel is valued for its strength, hardness, and beneficial mechanical properties, offered at an economical cost. Aluminum contributes a corrosion-resistant and visually appealing oxidized surface. This combination not only extends the durability of the steel but also broadens its usage across various applications, thereby harnessing both the robust nature of steel and aluminum's protective capabilities.
Products made of aluminized steel are distributed by steel service centers that receive the material from steel mills. The aluminizing process, akin to galvanizing, is conducted on regular steel to satisfy the requirements of downstream industries like construction, automotive, aerospace, transportation, shipbuilding, household appliances, HVAC systems, bakeware, and fireplaces.
Aluminized steel capitalizes on the advantages of both steel and aluminum. With a density and weight 250% greater than aluminum, steel exhibits much greater strength. However, steel left uncoated is susceptible to rust and corrosion when exposed to environmental factors. Aluminum, with its naturally occurring protective layer, shields against rust and corrosion, thereby making it an exceptional match for steel in aluminized steel products.

Aluminum is one of the most commonly used metals in industrial manufacturing and engineering today. As a lightweight metal, aluminum offers exceptional versatility for a variety of applications, ranging from aerospace and automotive engineering to construction and electronics. Aluminum coatings are especially valued for their outstanding protective and functional qualities. Among these, the high strength-to-weight ratio stands out, making aluminized components ideal for use in automobiles, aircraft parts, and structural applications where weight reduction without sacrificing durability is essential. In addition to being lightweight, aluminum and its surface coatings deliver many desirable surface characteristics, which contribute to their widespread use as protective metal coatings.
The primary advantage of aluminum coatings is their remarkable corrosion resistance, which extends the service life of metal substrates in aggressive environments. Other important properties, such as electrical conductivity, thermal efficiency, toughness at low temperatures, high reflectivity, non-magnetic qualities, and non-sparking attributes, further enhance their suitability for modern industry. This makes aluminum coatings a top choice for users seeking materials offering both performance and value in metal finishing, surface protection, thermal management, and more. Explore these key features below to better understand why aluminum coatings are such a reliable solution across multiple sectors.

Corrosion resistance is a key attribute of aluminized steel and aluminum-coated products, making them highly sought after in environments ranging from marine applications to chemical processing plants. Aluminum inherently possesses high resistance to corrosion, effectively shielding the underlying metal from two primary mechanisms: direct chemical attack and electrochemical action. This makes aluminum coatings critical for environments where exposure to moisture, salt, acids, and harsh chemicals is common.
Direct chemical attack, also known as pure chemical corrosion, occurs when a highly reactive agent comes into direct contact with the bare surface of a metal. This spontaneous reaction produces liquid and gaseous byproducts that escape or disperse from the corrosion site, while solid byproducts, such as rust or metal oxides, remain. Over time, the accumulation of these metal oxides on the surface can help to protect the underlying metal from further corrosion. This process is particularly important for aluminum-clad surfaces exposed to harsh industrial or coastal atmospheres.
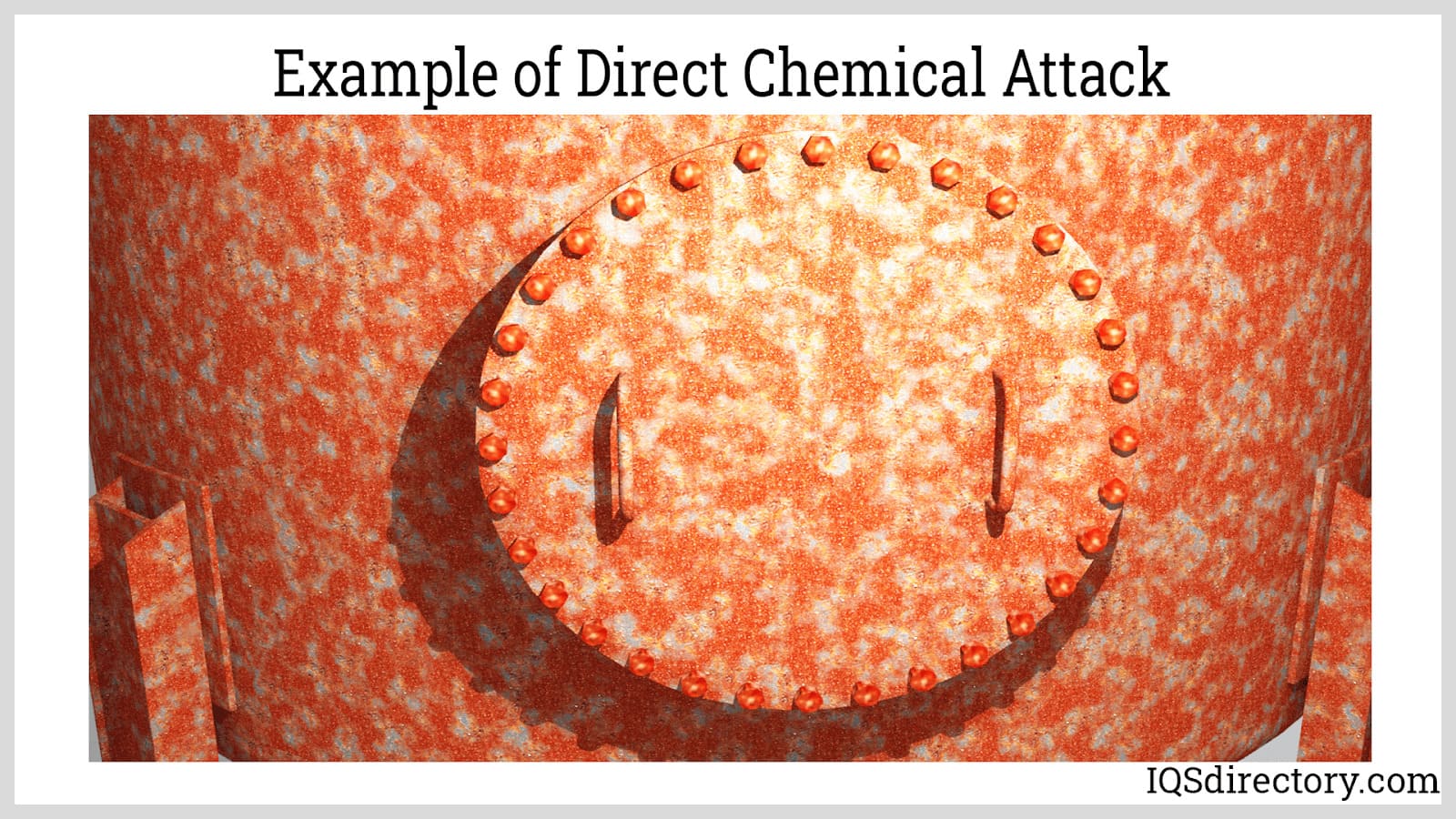
Aluminum resists corrosion effectively due to the formation of a thin, protective layer of aluminum oxide on its surface. This oxide layer, created by a direct chemical reaction, seals the metal from oxygen, water, and corrosive agents, preventing further degradation of the material. It forms almost instantly upon exposure to the atmosphere and can regenerate if damaged, ensuring nearly permanent corrosion resistance and low maintenance over time. This makes aluminum coatings ideal for long-term corrosion prevention in both indoor and outdoor environments.
Another protective mechanism of aluminum is its electrochemical behavior. Electrochemical corrosion occurs when an electrolyte solution connects the metal with a corrosive agent, such as acids or cations from less active metals.
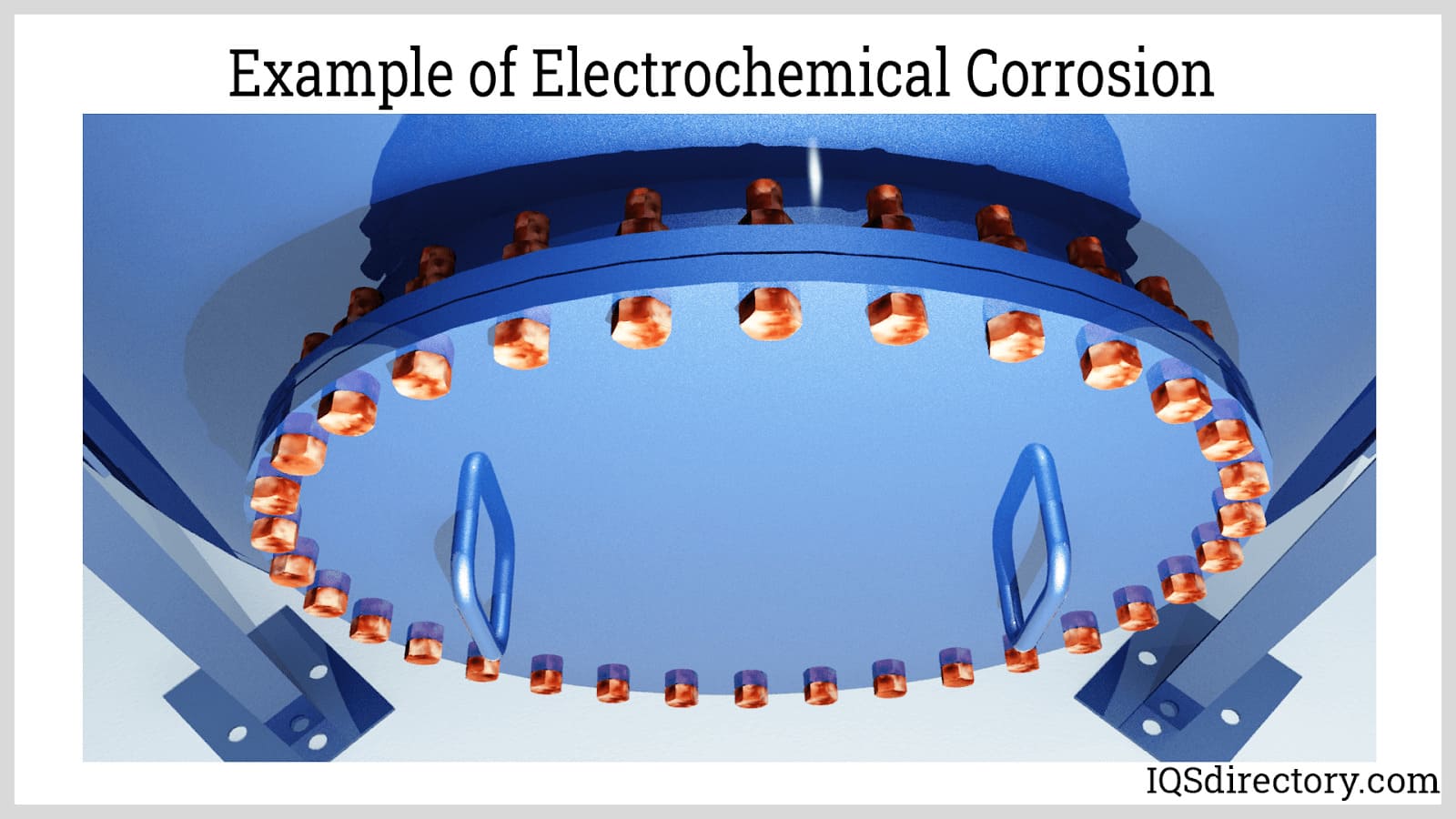
Typically, the aluminum oxide layer provides sufficient corrosion resistance. However, in situations where this layer cannot regenerate, such as in highly acidic or alkaline environments, the underlying aluminum alloy still offers protection against electrochemical corrosion. During such reactions, the aluminum coating, being highly active, corrodes instead of the steel. Thus, aluminum acts as a sacrificial anode, providing cathodic protection. It is an effective anodic material, comparable to zinc, commonly used in galvanic coatings and protective finishes.
These unique anti-corrosive mechanisms make aluminum coatings indispensable for projects demanding longevity and reliability. Industries such as construction, HVAC, marine, and automotive manufacturing frequently specify aluminized steel or aluminum-plated components to meet stringent corrosion resistance standards, reduce life-cycle costs, and ensure operational safety.
Aluminum offers about 61% of the electrical conductivity of copper. Despite this lower conductivity, aluminum is often preferred for certain electrical transmission, power distribution lines, and conductor applications because of its much lower density and cost-effectiveness. Aluminum's lightweight properties allow for easier installation and reduced structural load in overhead high-voltage transmission lines, making it an essential material for modern electrical infrastructure. Additionally, its resistance to oxidation ensures stable electrical performance over time.
Aluminum conducts heat at approximately twice the rate of brass and four times more efficiently than steel. This high thermal conductivity makes aluminum a popular choice for heat sinks, radiators, and other thermal management solutions in electronics, automotive, and aerospace industries. The material's ability to quickly dissipate heat contributes to improved energy efficiency and extended lifespan of critical components.

Unlike steel, aluminum maintains its toughness and ductility even at low temperatures, where other metals might become brittle and fail. The mechanical properties of aluminum remain relatively stable across a wide range of operating temperatures, making it a suitable choice for cryogenic storage tanks, refrigeration systems, and aerospace components exposed to sub-zero environments. This unique characteristic results in increased safety, performance, and design flexibility for manufacturers working within the most demanding conditions.
Aluminum's inherent toughness gives it high resilience and impact strength. It can absorb sudden forces or shocks and elastically flex under dynamic loads, reducing the likelihood of cracks or catastrophic failures. Because of these properties, aluminum is commonly specified in high-impact and load-bearing applications including vehicle body panels, structural frames, and safety equipment.
Aluminum is highly reflective, especially in the 200-400 nm range, surpassing the reflectivity of gold and silver. This makes aluminum coatings an optimal choice for use on glass to create household and automotive mirrors, energy-efficient light fixtures, and solar reflectors. With the right finish or surface treatment, aluminum can reflect approximately 90% of visible light and a significant amount of infrared and ultraviolet radiation, enhancing energy efficiency in buildings and industrial processes. The ability to fabricate highly reflective aluminum surfaces is critical in both optical and energy-saving technologies.

Aluminum is paramagnetic and does not exhibit ferromagnetism like steel. It doesn’t become magnetized under strong magnetic fields, making it ideal for electronic and electrical enclosures, components subject to EMI/RFI shielding, and sensitive instrumentation. Its non-magnetic characteristics help prevent magnetic interference in high-frequency or precision electronics, supporting compliance with electromagnetic compatibility (EMC) standards.
Aluminum, whether pure or alloyed, does not produce sparks, making it suitable for manufacturing tools used in hazardous, flammable, or explosive settings, such as oil refineries, chemical plants, and mining operations. Aluminum non-sparking tools are essential for protecting workers and equipment in locations with a risk of ignition from accidental impacts.
By selecting aluminum coatings or aluminized steel, businesses can benefit from a combination of corrosion protection, thermal and electrical conductivity, lightweight strength, and design versatility. These properties contribute to lower maintenance costs, energy efficiency, reduced structural load, and improved product performance. As a result, aluminum-coated products are used in roofing panels, HVAC ducts, heat exchangers, protective housings, and numerous other industrial and commercial applications.
When evaluating and purchasing aluminum coatings or finished parts, it's important to consider key factors such as coating thickness, adhesion quality, type of substrate, application environment, and the specific industry standards to be met. Working with reputable aluminum suppliers and manufacturers ensures quality assurance and compliance with technical requirements for your application.
Aluminized steel has emerged as a high-performance material, offering several advantages over traditional metals used for similar purposes. While stainless steel and galvanized steel are extensively utilized in industries that demand enhanced corrosion protection for structural integrity and longevity, aluminized steel is rapidly becoming a preferred alternative due to its distinctive combination of mechanical strength, thermal resistance, and cost effectiveness. In the sections below, we'll explore the core benefits of aluminized steel, compare it to other metal coatings, and highlight its relevance in numerous manufacturing and engineering applications.
Aluminized steels are engineered to shield against both direct chemical attack and electrochemical action. Direct chemical attack, often termed dry corrosion, can occur without the presence of an electrolyte and is typical in environments with high temperatures and industrial fumes. Electrochemical corrosion (wet corrosion), on the other hand, happens in the presence of moisture, resulting in material degradation. Aluminized steel’s dual-layer defense system offers reliable protection against both corrosion mechanisms, making it ideal for products exposed to atmospheric moisture, industrial processing, or outdoor elements.
Stainless steel is renowned for its impressive resistance to corrosion, owed in part to its chromium-rich oxide layer, but complications can arise when it is coupled with other metals such as carbon steel. This pairing often leads to galvanic corrosion, where the less noble metal (carbon steel, the anode) experiences accelerated corrosion when in contact with a more noble metal (stainless steel, the cathode), especially in electrolytic environments.
Aluminized steel avoids these galvanic corrosion issues due to its aluminum coating, which is more anodic than steel and therefore sacrificially corrodes, protecting the base metal. This sacrificial nature of the aluminum layer is essential in structures and components exposed to harsh, mixed-metal conditions such as those found in automobile manufacturing, HVAC units, and industrial piping systems. Other metals (magnesium, beryllium, zinc) are even more anodic and are sometimes incorporated into specialty coatings for enhanced performance.

Thanks to this anodic aluminum layer, galvanic corrosion is significantly minimized in aluminized steel. This makes the material especially valuable in applications where metal dissimilarity and electrical conductivity are factors, such as exhaust systems, chimneys, and architectural components. By reducing the risk of galvanic deterioration, aluminized steel contributes to extended service life and lower maintenance costs.
When compared to stainless steels, aluminized steels are considerably more affordable. This affordability is largely due to the use of plain carbon steel as the core substrate, with only a thin yet effective aluminum-based alloy coating (ranging from 30 to 270 grams per square meter, or 0.10 to 0.90 ounces per square foot). Unlike full-alloy stainless steel sheets, which require higher raw material and processing costs, aluminized steel optimizes resource efficiency without sacrificing protection.
In the realm of steel processing, multiple protective coatings exist to enhance performance under demanding conditions. Popular methods include anodizing, electroplating, and powder coating, each providing unique surface characteristics. The hot-dip aluminizing process produces a metallurgically bonded aluminum-silicon layer, which offers superior resistance to rust, scale, and corrosion. Although hot-dipping involves specific operational costs, these are comparable to alternatives like galvanizing (zinc-coating of carbon steel) and other advanced steel treatments. Anodizing, electroplating, or adding metals such as copper, lead, or tin can be effective, but often at a higher price point or limited by specific application needs.
When evaluating cost, durability, and lifecycle, aluminized steel presents a compelling choice for industries producing water heaters, ductwork, oven panels, farm equipment, and more.
A key advantage of aluminized steel lies in its remarkable thermal stability, maintaining structural and mechanical integrity even when exposed to temperatures as high as 1292°F (700°C). The unique properties of the aluminum-silicon alloy coating yield exceptional heat resistance while minimizing scale formation and oxidation, which can critically degrade lesser steel grades. This makes aluminized steel a top-tier selection for manufacturing components such as heat exchanger tubes, automotive exhaust systems, furnace baffles, water heater tanks, and other equipment regularly subjected to continuous thermal cycling.

By comparison, the performance of stainless steels can be grade-dependent. Specialty alloys like 316Ti, doped with elements such as titanium or niobium, offer improved heat stability, but standard stainless steel grades can be prone to oxidation and carbide precipitation (intergranular corrosion) above 932°F (500°C). This limitation stands in contrast to aluminized steel, which is specifically tailored for sustained high-temperature exposure.
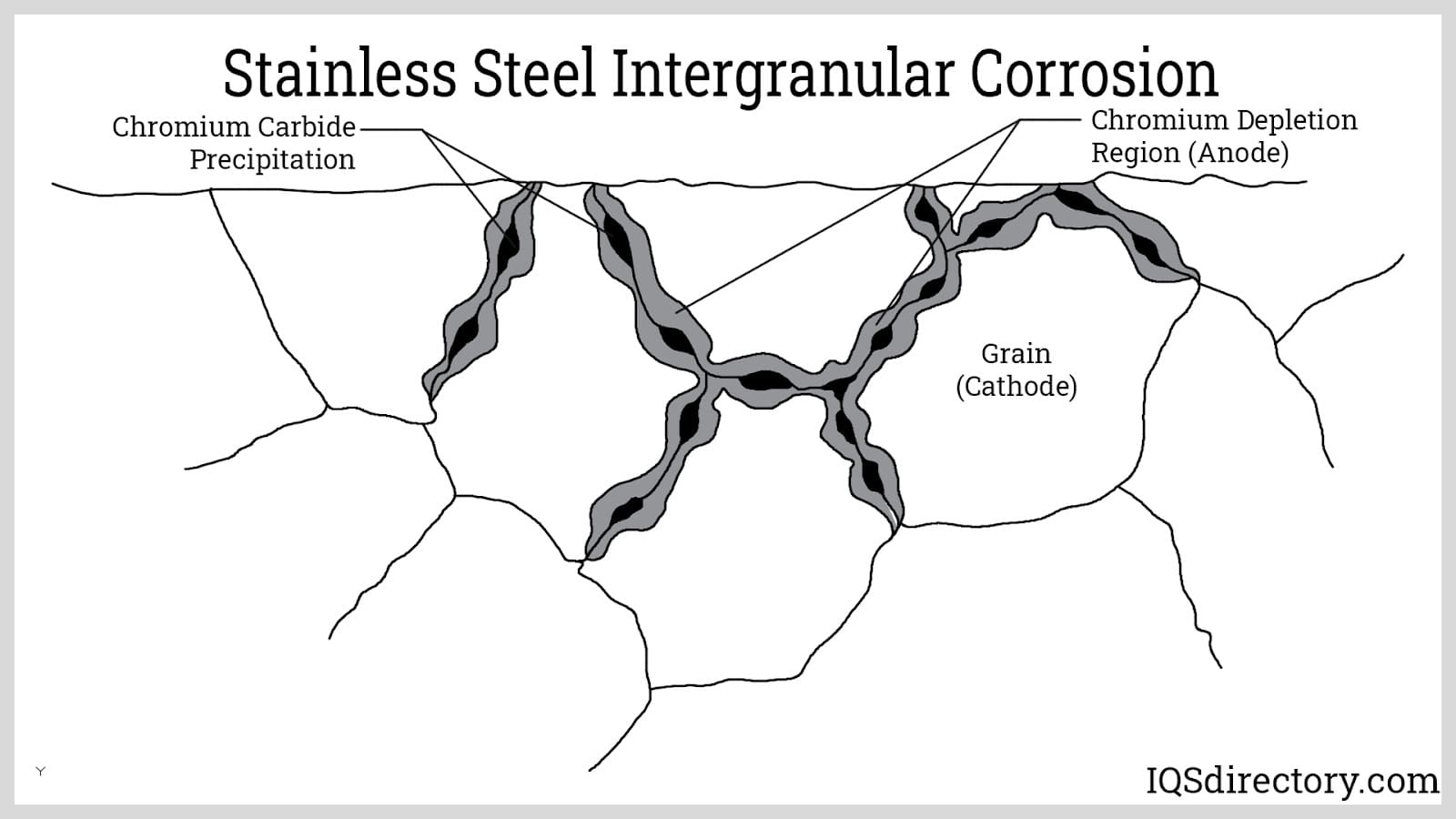
Galvanized steels, on the other hand, are typically rated for moderate temperatures (up to approximately 482°F or 250°C), after which the zinc coating becomes unstable, leading to iron-zinc alloy formation and eventual coating failure. For industrial ovens, furnace assemblies, and automotive exhausts, aluminized steel’s high heat endurance and oxidation protection stand out among available coated metal sheet options.
Another significant benefit of aluminized steel is its superior heat reflectivity. Aluminum naturally reflects a substantial portion of both visible and infrared radiation, making aluminized steel ideal for applications where minimization of radiant heat transfer is critical. With a high-reflectivity surface capable of bouncing back up to 80% of incoming infrared energy (depending on surface quality and finish), aluminized steel is widely used in thermal shielding, building insulation materials, oven panels, and components requiring energy-efficient heat management.
Enhanced heat reflectivity not only translates to improved thermal insulation but also contributes to energy conservation, reduced operating temperatures, and lower risk of heat-related material degradation. These characteristics make aluminized steel highly desirable for projects involving heat exchangers, furnaces, radiant barriers, and specialty cookware.
Beyond its technical strengths, aluminized steel offers excellent versatility for manufacturers and fabricators. It is highly formable and weldable, adapting to a wide range of fabrication techniques including stamping, rolling, and bending, all while maintaining its protective surface features. The aluminum coating lends an appealing, bright finish, making it suitable for visible applications in appliances, kitchenware, and architectural panels. Furthermore, aluminized steel is resistant to scaling, soot accumulation, and discoloration, ensuring low maintenance and longer aesthetic lifespan.
In summary, aluminized steel is a multipurpose, high-performance solution for any industry seeking optimal corrosion protection, heat resistance, cost efficiency, and visual appeal. Whether for HVAC systems, automotive exhausts, or equipment exposed to extreme temperatures and environmental conditions, aluminized steel delivers long-term value and material reliability for demanding engineering and construction projects.
Aluminized steel resists corrosion by combining steel's strength with an aluminum coating that forms a regenerative oxide layer, effectively shielding the steel from both direct chemical and electrochemical corrosion.
Aluminized steel offers strong corrosion protection, heat resistance up to 1292°F (700°C), and affordability. Unlike galvanized steel, it withstands higher temperatures, and compared to stainless steel, it is more cost-effective while maintaining comparable durability.
Aluminized steel maintains structural strength in high-heat environments, reflects up to 80% of infrared energy, and resists oxidation and scale, making it ideal for exhaust systems, ovens, and insulation materials.
Industries such as construction, automotive, HVAC, marine, and manufacturing use aluminized steel for its durability, corrosion resistance, heat tolerance, and attractive finish in demanding and high-performance applications.
Its dual-layer aluminum coating protects against both wet and dry corrosion while withstanding temperatures up to 1292°F (700°C), ensuring longevity in challenging settings such as HVAC systems and exhaust components.
Yes, steel service centers across the US distribute aluminized steel, supplying materials tailored for construction, automotive, HVAC, and various industrial uses.
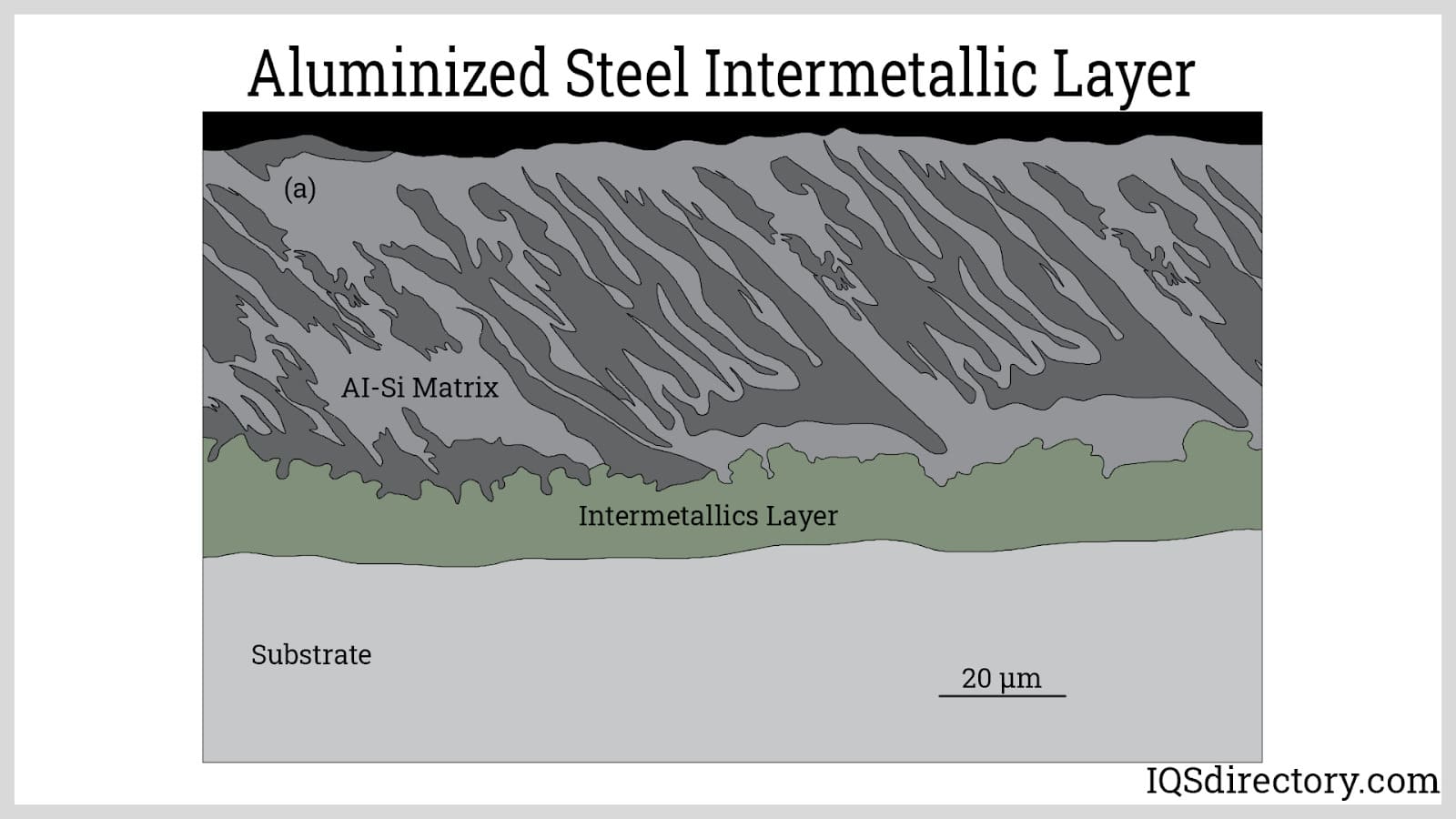
Hot-dipped aluminized steel is categorized into two primary grades based on the composition of the aluminum bath. The first grade, known as Type 1, consists of an aluminum-silicon alloy. The second grade, Type 2, is made from pure aluminum. Each type of aluminized steel exhibits distinct properties tailored for different applications.
Type 1 aluminized steel is hot dipped to form a thin layer of aluminum with 5% to 11% silicon added to create better adhesion of the aluminum layer. Silicon helps in the formation of a brittle intermetallic layer between the thin outer aluminum coating and various types of steel base metals.
Silicon slows the growth and controls the formation of the intermetallic layer, which improves the heat resistance and workability of aluminized steel. Although the addition of silicon is beneficial in enhancing the bonding of the aluminum layer and the base steel, it does slightly deteriorate its corrosion resistance and electrical conductivity and creates black spots on the finished aluminized steel. Type 1 aluminized steel is typically used in industrial equipment and commercial products such as mufflers, furnaces, ovens, ranges, heaters, water heaters, fireplaces, and baking pans.

The molten aluminum bath of type 2 aluminized steel is composed of commercially pure aluminum. It is designed for use in conditions where the primary requirement is resistance to atmospheric corrosion. Type 2 aluminized steels are used as structural materials for enclosures, sewage piping, corrugated roofing, siding, grain bins, drying ovens, and air conditioner condenser housings.

Aside from carbon steel, several types of steel are used in the aluminizing process. The positive properties of aluminum are ideal for the protection of all steels, including commercial grade, forming, deep draw, structural, and high-strength low alloy steels, all of which are susceptible to the same negative effects as carbon steel. Applying aluminum alloy increases the longevity of the steels and the number of applications for which they can be used.

Commercial steel, also known as mild steel or commercial quality steel, is widely used for general-purpose applications. It is characterized by its ductility, softness, and malleability, allowing it to be bent in any direction without cracking. This steel typically has a carbon content of approximately 0.10%.
Forming steel is distinguished by its lower carbon content compared to commercial steel, making it more ductile and malleable. It contains between 0.02% and 0.10% carbon.
Deep Drawing Steel is used in processes where the metal is radially drawn from a forming die. These steels have a carbon content of about 0.06%.
Extra Deep Drawing Steel is similar to Deep Drawing Steel but features an even lower carbon content, around 0.02%, which enhances its ductility.
Structural Steel is an industrial-grade steel with a carbon content ranging from 0.20% to 0.25%, making it harder than commercial steel. Its high tensile strength makes it ideal for construction and building applications. This category includes various types such as carbon steel, high-strength carbon steel, weathering steel, and fire-resistant steel.
High-Strength Low-Alloy Steel is defined by its mechanical properties rather than specific chemical compositions. It typically has yield strengths ranging from 250 to 590 MPa.
Ferritic Stainless Steel, an alternative to austenitic stainless steel, is enhanced by the aluminizing process. It is a low-carbon, high-chromium stainless steel with minimal or no nickel content. Known for its ductility, corrosion resistance, and magnetic properties, Ferritic Stainless Steel belongs to the 400 series of stainless steels, categorized by their composition and applications.
Hot dipping is the primary method used to produce aluminized steel due to its cost-effectiveness and relatively simple equipment requirements. While hot dipping is the most common process, several alternative aluminum coating methods are also available. These methods are summarized and briefly explained below.
Calorizing is a surface treatment technique designed to apply aluminum coatings to steel, enhancing its resistance to heat and corrosion. In this process, steel is exposed to a mixture of aluminum powders within a furnace. The high temperatures in the furnace cause the aluminum to diffuse onto the steel's surface. Calorizing can be carried out using two main methods: pack diffusion and the slurry method.
Electroplating is a process used to deposit a thin metal layer onto an electrically conductive surface using two electrodes or conductors. In this process, the anode electrode, which is positively charged, causes metal ions to move toward the negatively charged cathode electrode, while the negatively charged ions return to the anode.
The metal to be coated is placed at the cathode, and the metal coating is positioned at the anode. As ions travel from the anode to the cathode, they adhere to and cover the steel workpiece. Direct current is applied to the anode, causing its metal atoms to oxidize and dissolve. When these ions reach the cathode, they are reduced and form a coating on the metal piece. The current is regulated so that the rate of metal dissolution at the anode equals the rate of plating at the cathode.
Electroplating, also known as electrodeposition, enhances the strength and durability of steel by applying an aluminum coating. It is commonly used in various industries, including aerospace, automotive, medical and dental tools, and microwave products.
Metal spray coating, also known as metalizing and thermal spraying, melts aluminum wire into a molten material and injects it into a compressed air stream, where the molten aluminum is atomized into fine droplets and sprayed onto the surface of the steel workpiece. The fine droplets quickly cool, fuse, and solidify to form a protective metallic layer or film with a thickness of 0.47 to 0.59 inches (12 mm to 15 mm).
For the metal spray coating process, the aluminum feedstock typically used is 85/15 wire. This method is designed to apply an aluminum coating to steel surfaces without forming an intermetallic layer, unlike hot dipping. However, metal spray coating often results in a less smooth finish compared to other coating methods, with surfaces appearing mottled, rough, and uneven.

Aluminum coating via the cladding process involves rolling, extruding, or drawing steel together with an aluminum sheet or film. This procedure is performed at elevated temperatures to ensure a strong bond between the aluminum coating and the steel component.
Hot dipping is a simple process comprising three primary steps: surface preparation, immersion, and finishing. To enhance the process, additional procedures such as flux coating and heat treatment may be included. The following sections provide a detailed explanation of each of these operations.
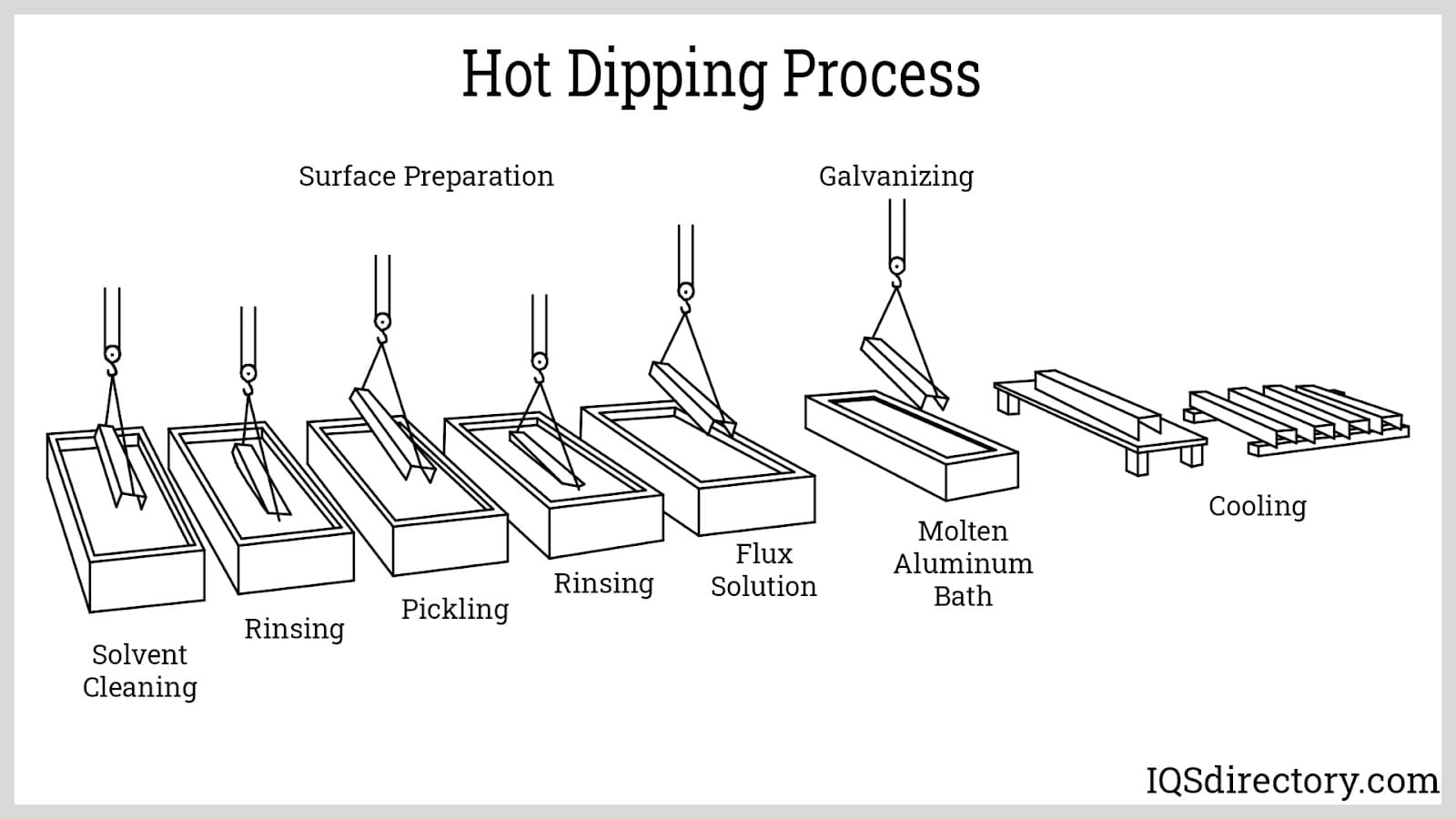
Fluxing: This process serves two purposes. The first is to prevent the oxidation of the surface of the workpiece after cleaning. Metals oxidize is faster at elevated temperatures. Since hot dipping occurs at the melting point temperature of aluminum alloy, the oxidation of the workpiece is accelerated. The newly created oxides can interact with the intermetallic layer and can decrease the bonding strength of the coating.
The second benefit of fluxing is to improve the wettingcharacteristics of the metal, ensuring that the whole surface is covered and free from voids or discontinuities. This further enhances the bonding strength of the intermetallic layer.
Aside from directly applying the flux coating onto the workpiece, fluxing can also be done by covering the molten aluminum bath with a flux solution. This is known as wet fluxing. Through wet fluxing, a layer of molten flux is applied to the surface of the aluminum bath. This not only prevents the workpiece from oxidizing upon contact with the molten bath but also prevents the bath from oxidizing.
Immersion: This is the main aluminizing process in which the workpiece is dipped into the molten aluminum bath. At first glance, this is a fairly easy process. However, certain difficulties arise, which can result in problems such as peeling of the aluminum coating, poor mechanical properties of the finished part, surface discontinuities, and workpiece deformation. To correct these, a few requirements must be followed.
The first and second requirements are easily met when steel is used as the base metal. Steel has a much higher melting point than aluminum. Also, both metals readily form alloys. The third requirement is based on the best practices of the manufacturer. Some variables that affect immersion time are the composition of the aluminum alloy, the target thickness of the intermetallic layer, and the molten bath temperature.

Steel service centers are companies that specialize in procuring steel directly from mills and manufacturers and supplying them to the customers. They are fundamental to the steel supply chain...
Aluminum 1100 is the softest of the aluminum alloys, which makes it easy to shape and form into a wide range of products for industrial and home use. It can be cold and hot worked but is frequently shaped by...

The term "aluminum coil" describes aluminum that has been flattened into sheets where their width is significantly higher than their thickness and then "coiled" into a roll. Stacks of individual aluminum sheets are difficult to...
Aluminum piping and tubing is silvery-white, soft, and ductile. The metal belongs to the boron group. Aluminum is the third most abundant element present on earth. Aluminum has low density. When exposed...
Beryllium Copper is a versatile copper alloy that is valued for its high strength and hardness, combined with good electrical and thermal conductivity. It is a non-ferrous, non-magnetic, and non-sparking metal alloy...

A variety of copper-zinc alloys are referred to together as brass. Different ratios of brass and zinc can be used to create alloys, which produce materials with various mechanical, corrosion, and thermal properties...
Copper is a ductile, malleable, and reddish-gold metal with the capacity to effectively conduct heat and electricity. Brass and bronze, two commonly used alloys, are created when copper is combined with...

The copper sheet is a highly malleable and workable metal with outstanding electrical and thermal conductivity and corrosion resistance. Copper (Cu) is a reddish, very ductile metal that belongs to Group 11 of the periodic table...

Metals are a group of substances that are malleable, ductile, and have high heat and electrical conductivity. They can be grouped into five categories with nickel falling in the category known as transition metals...

Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...

Stainless steel is a type of steel alloy containing a minimum of 10.5% chromium. Chromium imparts corrosion resistance to the metal. Corrosion resistance is achieved by creating a thin film of metal...

Stainless steel grades each consist of carbon, iron, 10.5%-30% chromium, nickel, molybdenum, and other alloying elements. It is a popular metal used in various products, tools, equipment, and structures that serve in many industrial, commercial, and domestic applications...

Stainless steel can be fabricated using any of the traditional forming and shaping methods. Austenitic stainless steel can be rolled, spun, deep drawn, cold forged, hot forged, or stippled using force and stress...

Stainless steel tubing is a multifaceted product that is commonly utilized in structural applications. Stainless steel tubing diameters and variations vary greatly based on the application requirements and are...

Titanium metal, with the symbol Ti, is the ninth most abundant element in the earth‘s crust. It does not occur in large deposits, yet small amounts of titanium are found in almost every rock...

Tungsten is a rare naturally occurring chemical element on earth. It is known to be one of the toughest metals on the earth. It is usually a tin white or a steel gray metal. Tungsten is common for its high tensile...
Aluminum is the most abundant metal on the Earth’s crust, but it rarely exists as an elemental form. Aluminum and its alloys are valued because of their low density and high strength-to-weight ratio, durability, and corrosion resistance...