Stainless Steel 304

Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...
Please fill out the following form to submit a Request for Quote to any of the following companies listed on
This article offers detailed information about stainless steel 316.
You will learn:

| Stainless Steel Grade 316 Spec Sheet | |
|---|---|
| 316 | 316L |
| ASTM A240 | ASTM A240 |
| ASTM A666 | ASTM A666 |
| ASME SA240 | ASME SA240 |
| AMS 5524 | AMS 5507 |
Stainless steel is a type of alloy composed of at least 10% chromium, a key element that gives it corrosion resistance. The chromium develops a protective oxide film on the metal's surface, shielding it from corrosive factors.
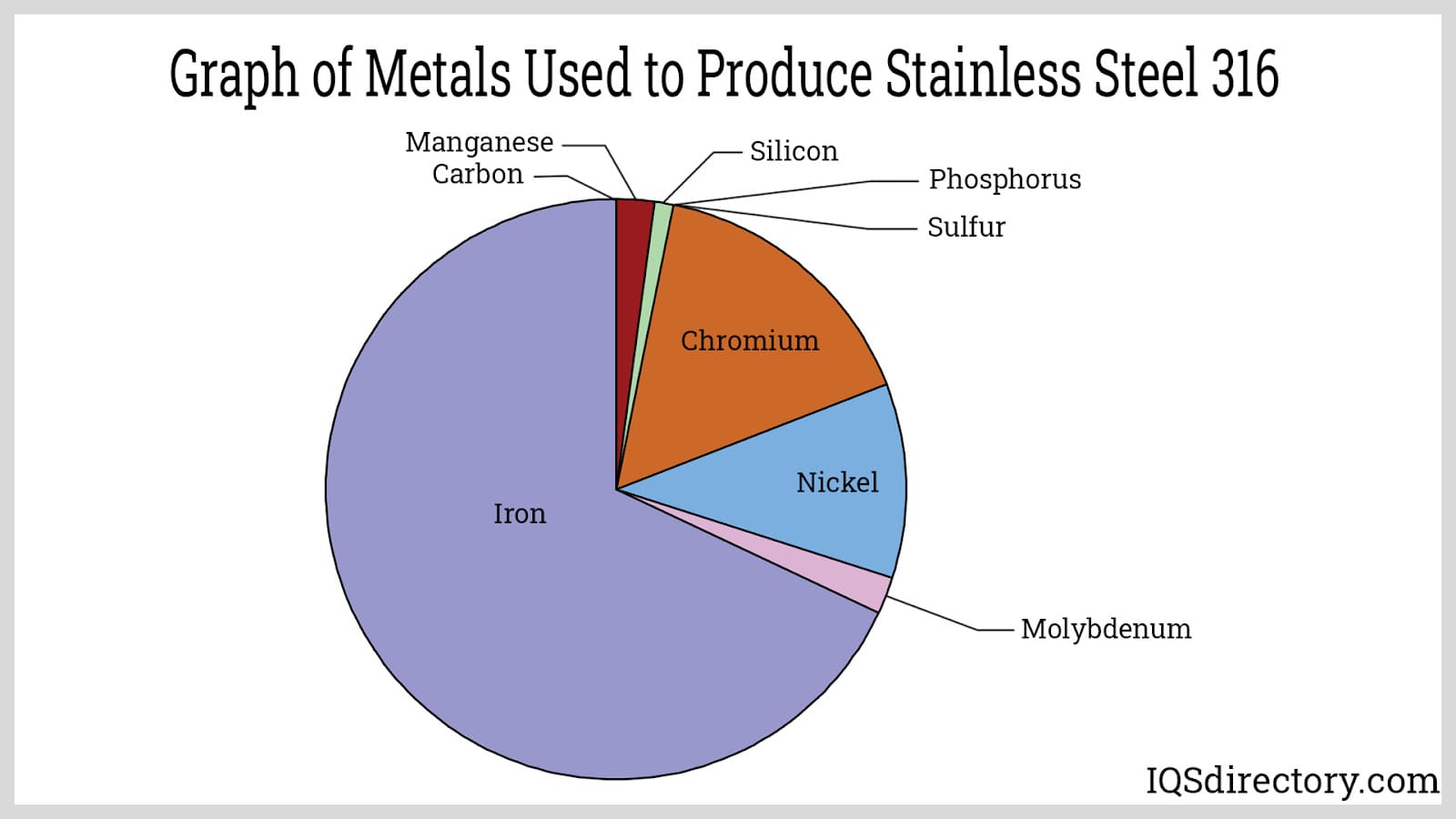
Among the various grades, stainless steel 316 is quite prevalent. It generally consists of 16 to 18% chromium, 10 to 14% nickel, 2 to 3% molybdenum, and traces of carbon. The inclusion of molybdenum enhances the corrosion resistance of stainless steel 316 when compared to other grades, with additional alloying improving its overall attributes.
The characteristics and advantages of stainless steel 316 make it the second most frequently used grade, right behind stainless steel 304. It is especially suitable for corrosive settings like chemical plants, refineries, and marine applications.
Stainless steel 316L, a low-carbon variant, is favored in cases where sensitization could be a problem. In contrast, stainless steel 316H has an increased carbon content, offering better thermal stability and creep resistance. Another variant, stainless steel 316Ti, is stabilized for enhanced resistance to intergranular corrosion.
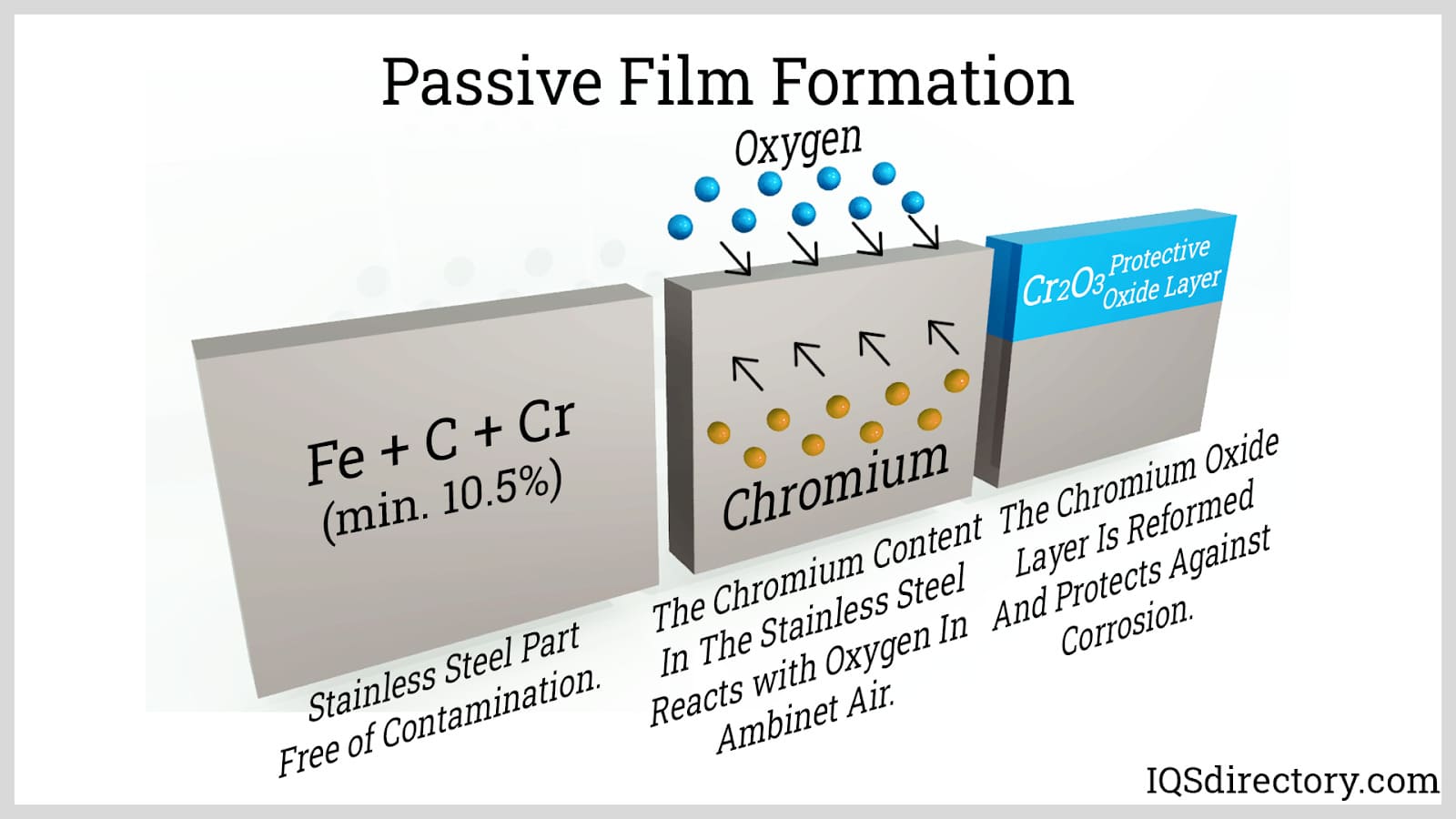
Stainless steel benefits significantly from passivation, a process that makes the metal "passive" or resistant to oxidation in corrosive environments and process fluids. This is accomplished by exposing the stainless steel to air, allowing the formation of a protective layer of chromium oxides on its surface.
To bolster the formation of the passive film, stainless steel is treated chemically in acidic passivation baths, which typically include nitric acid. This cleaning process eradicates impurities such as free iron or iron compounds that could compromise the passive layer.
After the acidic treatment, the metal is neutralized in a sodium hydroxide bath. Furthermore, a descaling procedure is used to remove oxide films that develop during high-temperature operations like hot-forming, welding, and heat treatment.
Stainless steel 316 distinguishes itself by its significant molybdenum content, imparting exceptional resistance to corrosion, particularly against chlorides and harsh chemical environments. Recognized as the second most widely used austenitic stainless steel after grade 304, stainless steel 316 offers advanced performance in demanding applications such as marine, pharmaceutical, food processing, and chemical processing industries. Austenitic stainless steels, such as 304 and 316, are characterized by their high nickel or nitrogen content, which provides a unique, non-magnetic crystalline structure and remarkable durability at a range of operating temperatures.
Stainless steels are systematically grouped based on their chemical composition, mechanical properties, metallurgical structure, and their intended industrial applications. The four primary families are: ferritic, martensitic, austenitic, and duplex stainless steels. Duplex stainless steels represent a hybrid classification, combining properties of two or more parent families—such as martensitic-ferritic or austenitic-ferritic—resulting in alloys with balanced mechanical strength and corrosion resistance. Each family’s fundamental matrix structure directly influences its performance capabilities, making the selection of the proper grade crucial for specific engineering and manufacturing requirements.
Within these families, stainless steel is further subdivided into grades to precisely describe the properties and chemical makeup of each alloy. Legacy grades are assigned three-digit numbers by the Society of Automotive Engineers (SAE), while alternative classification systems—such as the six-digit ASTM/UNS system used in North America—provide additional detail. Regardless of the numeric system, each stainless steel grade must strictly comply with its specified alloy composition and regulated content of elements like chromium, nickel, carbon, and molybdenum, as these directly impact corrosion resistance, mechanical strength, and other key properties. Even minor modifications in alloying elements can cause significant changes in how a stainless steel grade performs, especially in aggressive or specialized environments.
For engineers and purchasers evaluating different types of stainless steel, understanding these grade variations is essential. Different grades offer diverse levels of corrosion resistance, tensile strength, ductility, hardness, temperature stability, and wear resistance. The primary factor influencing these characteristics is the alloy’s microstructure, visible under a microscope (often at 25x magnification). The microstructure—determined by the ratios and types of alloying elements—dictates suitability for applications such as construction, medical devices, foodservice equipment, and chemical storage tanks.

Within stainless steel 316, the refined cellular microstructure features boundaries enriched with chromium, manganese, molybdenum, and niobium. This microstructural enrichment fosters its enhanced corrosion resistance and pitting resistance, especially in acidic or chloride-rich settings like seawater, desalination plants, and chemical production. The fine distribution of chromium and molybdenum along the boundary zones has a direct impact on the alloy’s durability and performance longevity, even under continuous exposure to harsh industrial chemicals or saline environments.

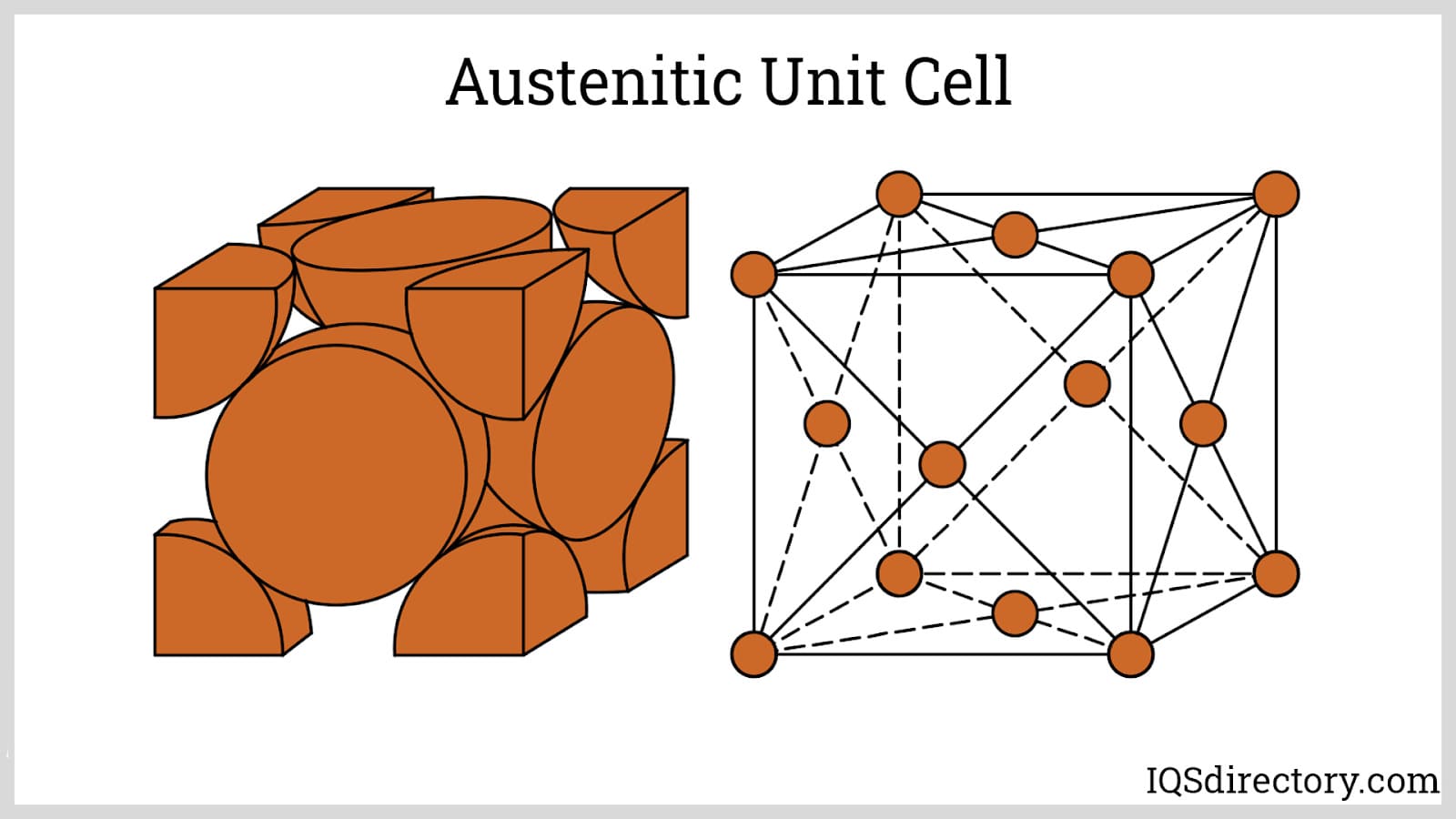
Austenitic Stainless Steels: The austenitic stainless steel family stands out for its non-magnetic properties, high chromium and nickel content, excellent ductility, and low levels of carbon—making these alloys the most popular choice globally. Used extensively in sectors demanding superior corrosion resistance and weldability, such as architecture, food processing, and pharmaceuticals, the austenitic grades also maintain their strength at both elevated and cryogenic temperatures.
The hallmark of austenitic stainless steels is their face-centered cubic (FCC) crystal structure, developed through the addition of nickel and, in some cases, nitrogen. This grain structure guarantees superior impact toughness and flexibility, vital for high-stress environment use. Notably, these steels retain steady mechanical properties across a wide range, offering outstanding formability and the ability to be fabricated into complex shapes or components.
Austenitic stainless steels cannot be hardened by heat treatment; instead, they are work-hardened through cold working processes, enhancing their strength, hardness, and resistance to cracking or deformation. Their innate non-magnetic character is maintained after annealing but some cold-working may induce partial magnetism, which is a key consideration for buyers seeking non-magnetic materials for critical components.
The principal alloying element in the 300 series austenitic stainless steels—including grades 304, 304L, 316, and 316L—is nickel, which typically ranges from 6% to 20%. Grade 316, for example, is known as a Cr-Ni-Mo system because of its additional 2-3% molybdenum content. Series 200 stainless steels, in contrast, contain lower nickel and higher nitrogen, resulting in a slightly reduced corrosion resistance profile and changes in mechanical properties. The selection between 200 and 300 series should be based on required corrosion resistance, cost, and specific application environment.
In direct comparison, the key factor separating stainless steel 304 and 316 is series 316's molybdenum content. This not only heightens resistance to localized corrosion such as pitting (especially in the presence of chlorides, phosphoric acid, or acetic acid) but also bolsters heat and wear resistance. As a result, 316 offers greater versatility in aggressive industrial and marine environments where exposure to saltwater, bleach, or acidic solutions can jeopardize material integrity. For projects seeking both strength and corrosion resistance, particularly with exposure to harsh chemicals or seawater, 316 is often the optimal stainless steel choice.
Want to choose between stainless steel 304 vs. 316 for your next project? Consider key criteria such as corrosion risk (chloride or salt exposure), required tensile strength, fabrication methods (weldability, formability), service temperature, and budget. Suppliers and stainless steel manufacturers can help you evaluate material certifications and compliance (such as ASTM, ASME, and ISO standards) relevant to your industry.
| Comparison of the Elements of Series 304 Stainless Steel and Series 316 Stainless Steel | ||
|---|---|---|
| Type 304 | Type 316 | |
| Carbon | 0.08% Max | 0.08% Max |
| Manganese | 2.00% Max | 2.00% Max |
| Phophorus | 0.045% Max | 0.045% Max |
| Sulfer | 0.030% Max | 0.030% Max |
| Silicon | 1.00% Max | 1.00% Max |
| Chromium | 18.00 - 20.00 | 16.00 - 18.00 |
| Nickel | 8.00 - 10.50% | 10.00 - 14.00 |
| Molybdenum | - | 2.00 - 3.00% |
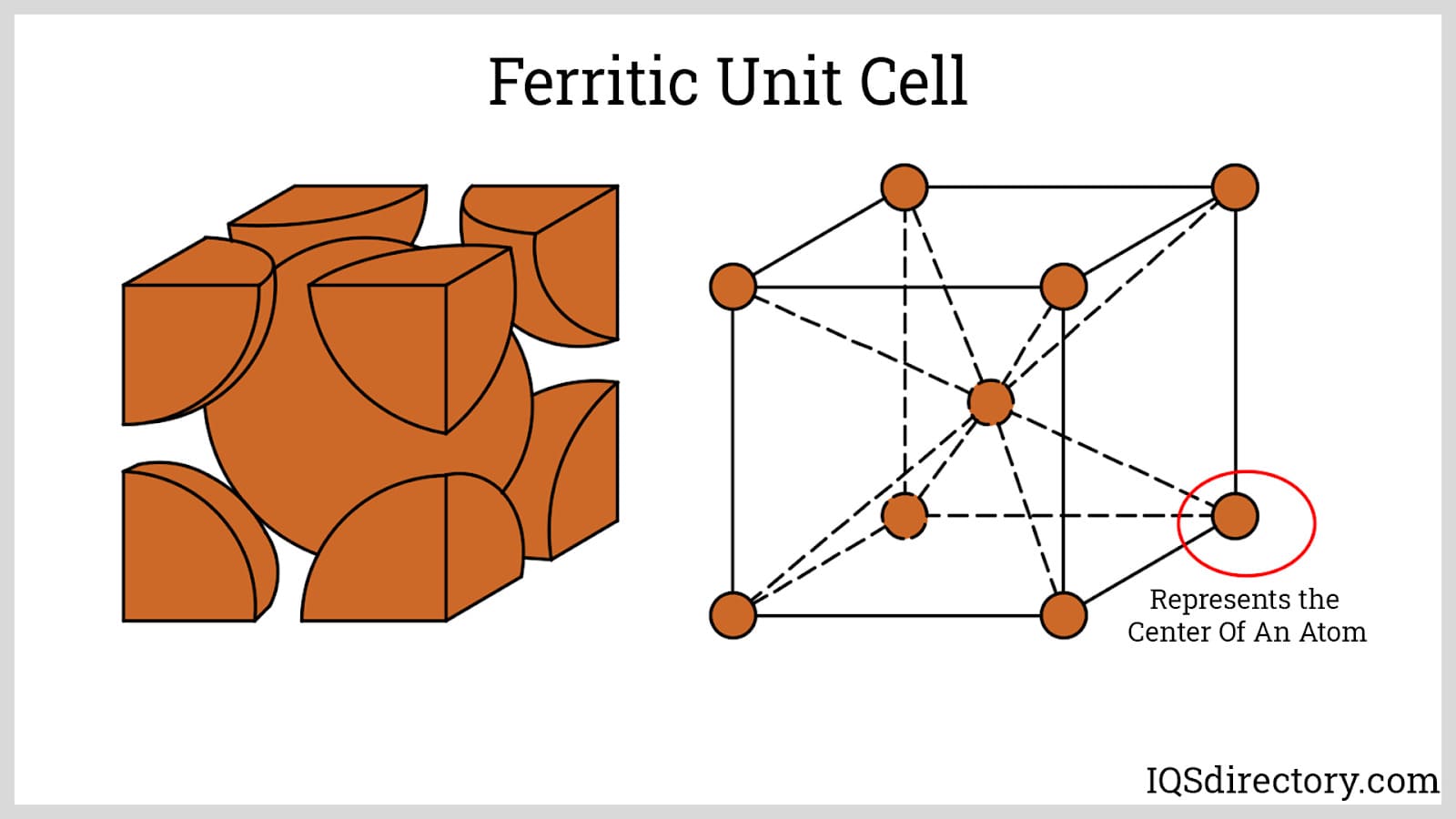
Ferritic Stainless Steels: Ferritic stainless steels exhibit a body-centered cubic (BCC) microstructure, stabilized by high chromium content with negligible amounts of nickel. This consistent ferritic phase renders them inherently magnetic and resistant to scaling at elevated temperatures, but more challenging to weld than austenitic grades due to grain growth and intermetallic precipitation at high temperatures. These alloys are typically specified under the AISI 400 series designation, and are favored in applications that require good resistance to stress corrosion and oxidation, such as automotive exhaust systems, industrial equipment, and kitchenware.
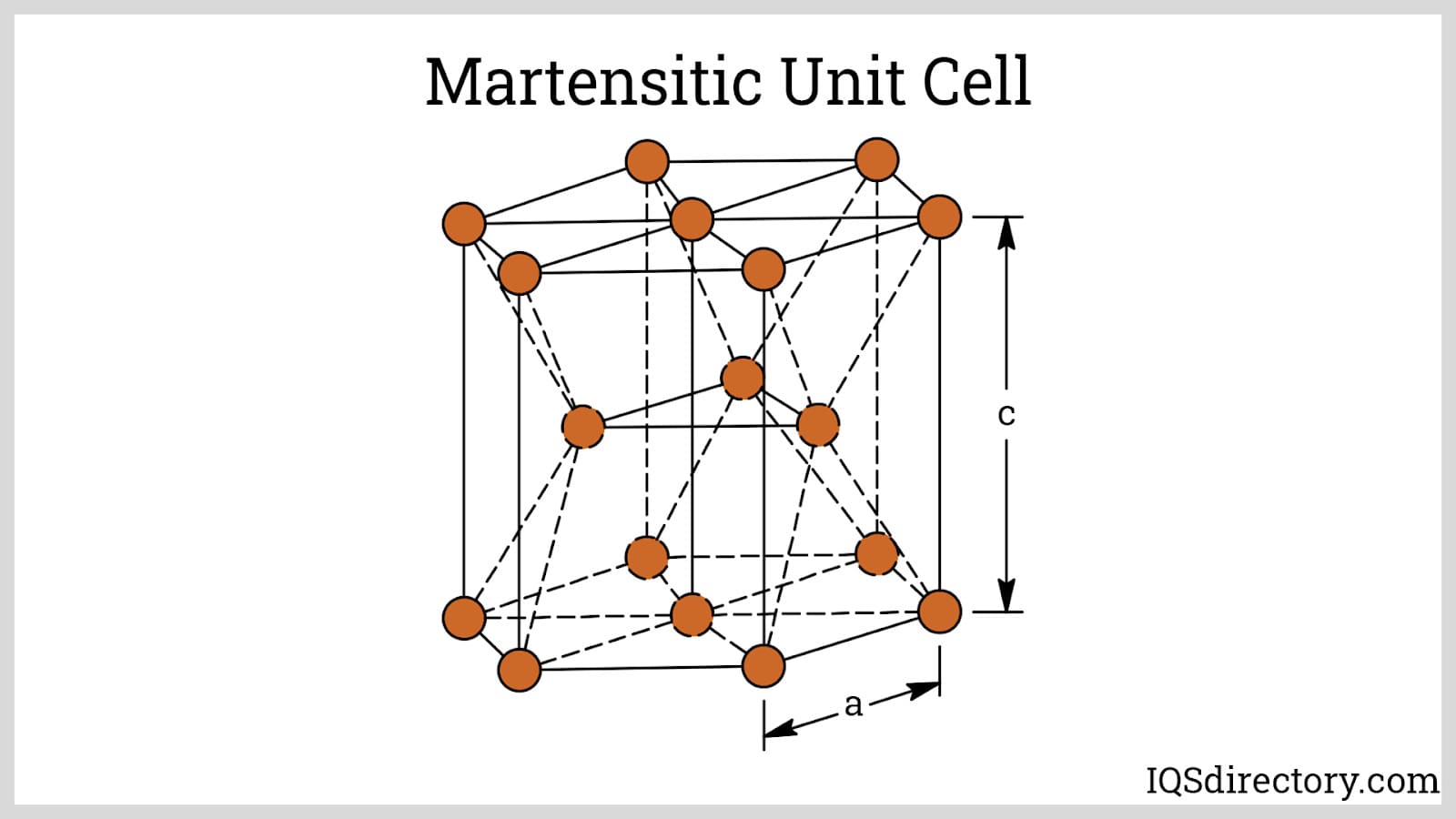
Martensitic Stainless Steels: Martensitic stainless steels are uniquely defined by higher carbon content, which allows them to be hardened through heat treatment. When rapidly cooled (quenched) from the austenitic phase, they develop a martensitic microstructure that is strong and hard but less ductile, making them excellent candidates for tools, blades, turbine components, and wear-resistant parts. Adjusting the carbon concentration enables a wide spectrum of hardness and strength profiles, though this comes at the expense of some corrosion resistance compared to other families. Like ferritic steels, martensitic stainless is commonly identified within the AISI 400 series.
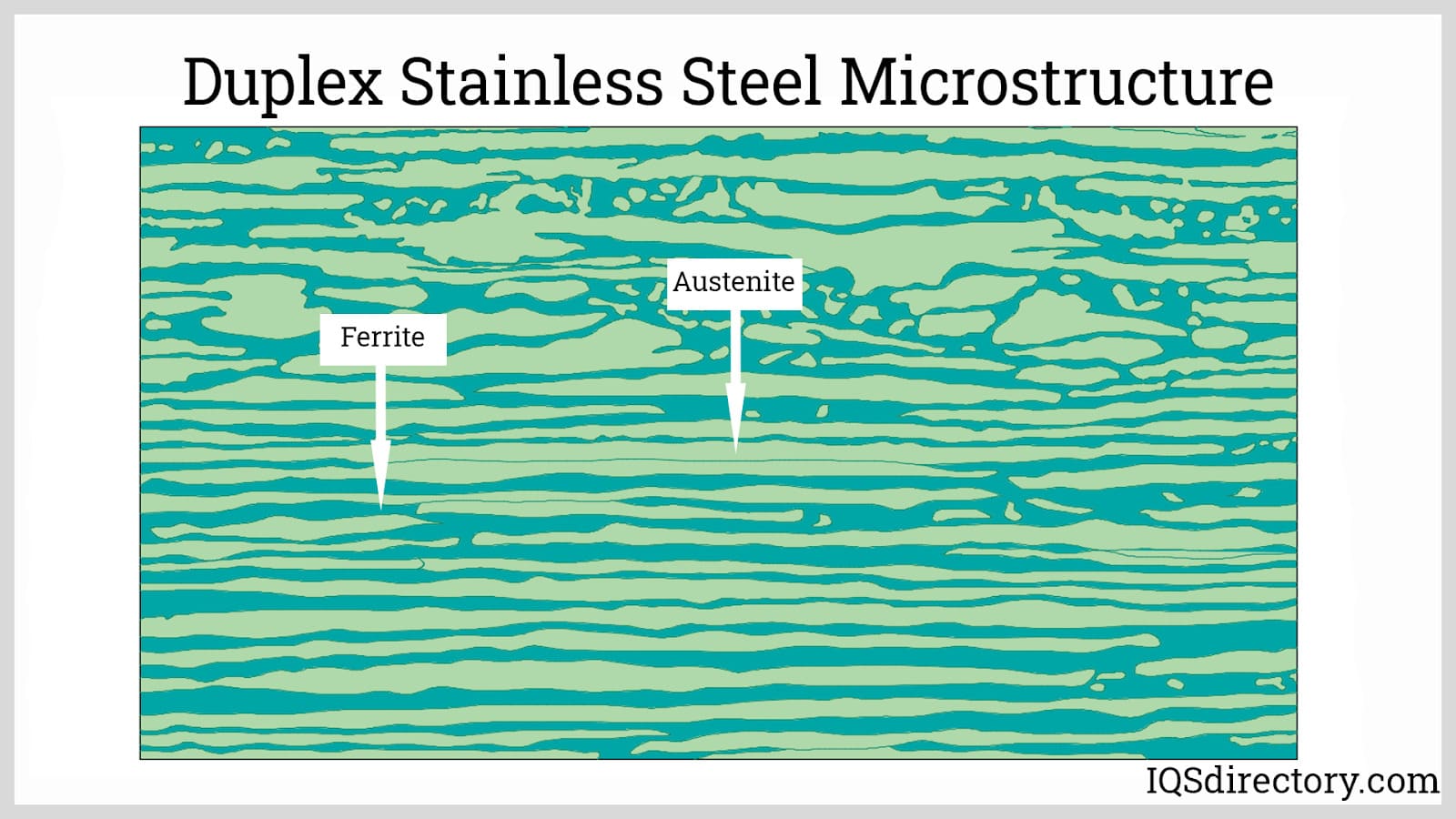
Duplex Stainless Steels: Duplex stainless steels combine approximately equal proportions of austenitic and ferritic microstructures, achieved by carefully balancing chromium and nickel content—often with added molybdenum or nitrogen. This dual-phase composition offers a unique combination of higher strength and improved resistance to stress corrosion cracking, especially in chloride-rich or caustic environments. Their balanced properties make duplex grades ideal for oil and gas, desalination, chemical processing, and structural applications demanding both mechanical strength and superior corrosion resistance. The most prevalent grade is duplex 2205 stainless steel, which is widely available from global stainless steel suppliers.
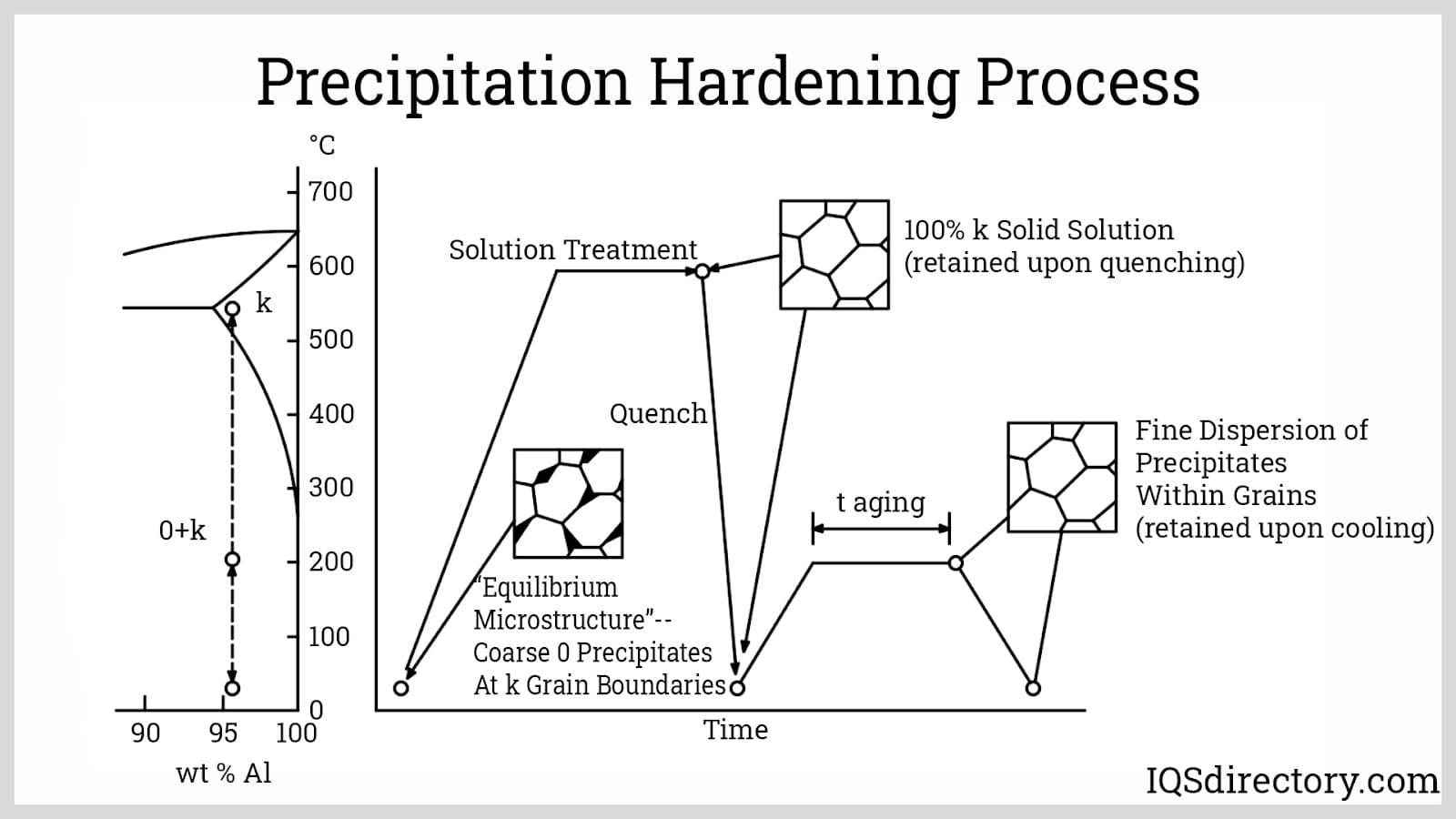
Precipitation Hardening Stainless Steels: These highly specialized stainless steels are designed for further strengthening through precipitation hardening—a heat treatment process that introduces fine secondary phase particles into the metal’s structure. Elements like copper, niobium, aluminum, and titanium are used to form these precipitates, refining the alloy’s mechanical performance without sacrificing essential corrosion resistance. Precipitation hardening grades are typically employed in aerospace, nuclear, and high-performance engineering applications where a balance of strength, toughness, and corrosion resistance is paramount.
Choosing the Right Stainless Steel Grade for Your Application
When selecting between stainless steel grades—such as 304, 316, duplex 2205, or specialty alloys—consider environmental exposure, required mechanical properties, industry standards, and cost factors. Thoroughly review the alloy certifications and consult with trusted stainless steel suppliers and manufacturers to ensure compliance with regulations (like ASTM or ISO) and suitability for your specific use case. By understanding the distinct characteristics and applications of each stainless steel family and grade, purchasers and engineers can confidently source the optimal material to maximize performance, safety, and value in their projects.
Source 21, Inc. is a leading stainless steel 316 supplier specializing in the distribution of stainless steel coils and strips, including both 316 and 316L stainless steel grades. Their extensive processing capabilities encompass coil cutting to length, surface polishing for aesthetic and corrosion resistance, masking for enhanced protection, as well as tempering for optimal mechanical strength. Source 21 supplies finished stainless steel products packaged in custom boxes, export crates, or specialized skid mounts, catering to the unique requirements of their clients. Major industries served include aerospace, roll forming, automotive manufacturing, and food processing equipment fabrication. By offering custom stainless steel solutions—from standard grades to premium alloys—Source 21 supports applications where durability, formability, and resistance to harsh environments are critical. Their commitment to precision processing and customer-focused service makes them a preferred partner for manufacturers seeking high-performance stainless steel 316 for structural, hygienic, and high-temperature uses.
Cleveland-Cliffs Inc. stands out as a leading producer of flat-rolled stainless steel in North America. With integrated operations that include iron ore mining in Michigan and Minnesota, the company maintains superior quality control throughout the stainless steel production chain. Cleveland-Cliffs produces all five principal types of stainless steel, including widely used 304, 304L, and high-corrosion resistance 316 stainless steel. Their products are engineered for demanding environments, making them a preferred source for automotive manufacturing, industrial fabrication, and construction. Their commitment to steel recycling, sustainability, and environmentally responsible manufacturing processes aligns with modern industry standards. Cleveland-Cliffs serves a broad clientele, offering custom specifications and material certifications that support diverse user requirements for strength, formability, and corrosion resistance, especially in sectors requiring reliable marine-grade alloys and cut-edge technology.
Allegheny Technologies (ATI) is a renowned specialty metals manufacturer, producing a wide variety of stainless steel grades, including premium austenitic, ferritic, superaustenitic, and superferritic alloys. ATI's stainless steel portfolio includes high-performance materials engineered for exceptional resistance to corrosion, oxidation, and high temperatures. They utilize advanced processes such as hot rolling and precision finishing, enabling them to deliver stainless steel in a broad range of thicknesses and dimensions suitable for critical applications. ATI is recognized for rapid order fulfillment and short lead times, which is valuable for industries requiring immediate supply, like energy, chemical processing, aerospace, and heavy equipment manufacturing. Their innovative approach ensures their products are ideal for food-grade equipment, medical instruments, and architectural components, reinforcing ATI’s reputation as a solutions provider of high-quality 316 stainless steel and related alloys.
Acerinox S.A., headquartered in Spain, is an internationally respected manufacturer of stainless steel products for a variety of industries. With a diversified production portfolio that includes stainless steel plates, hot and cold-rolled coils, sheets, strips, and discs, Acerinox delivers to customers serving the transportation, industrial equipment, food processing, and environmental technology sectors. Their focus on innovation, material consistency, and global distribution ensures that clients can specify stainless steels—like the versatile 316 grade—in the form, finish, and tolerance that best suits sophisticated engineering and manufacturing needs. The company's advanced metallurgy, rigorous quality assurance, and strong export capabilities make it a favored supplier among businesses seeking international stainless steel sourcing solutions for applications where corrosion resistance, sanitation, and long service life are non-negotiable.
Aperam is a globally recognized supplier of high-performance stainless steel 316 and 316L products, delivering materials to more than 40 countries. With an annual production capacity of 2.5 million tons spanning five cutting-edge facilities, Aperam manufactures coils, sheets, tubes, discs, flat bars, strips, and heavy plates that meet stringent international standards. Their Sterling Heights, Michigan service center specializes in flat stainless steel, offering tailored solutions for industries ranging from architectural construction and automotive to chemical processing and renewable energy. Aperam emphasizes sustainability through eco-conscious manufacturing and the use of recycled materials, with a notably low CO2 footprint. Their commitment to continual innovation, technical support, and traceable product quality positions Aperam as a trusted supplier of advanced stainless steel 316 products for customers prioritizing environmentally responsible sourcing alongside high mechanical and corrosion performance.
When selecting a stainless steel 316 manufacturer or distributor, it is essential to consider factors such as certified quality processes (including ISO 9001 accreditation), availability of custom fabrication services, technical support, and fast delivery options. Users in industries like pharmaceutical processing, food handling, marine construction, and petrochemical applications frequently require materials traceability, specialized surface finishes, and documentation to comply with regulatory standards. Innovative suppliers also offer value-added solutions like precision slitting, laser cutting, welding, and component assembly, enabling customers to streamline production efficiency and minimize waste. To determine the best fit for your project, compare suppliers based on their range of 316 and 316L stainless steel inventory, responsiveness to inquiries, and proven track record in supporting project-specific requirements.
Stainless steel 316 contains 2-3% molybdenum, providing superior resistance to corrosion, especially against chlorides and harsh chemicals. This makes it the preferred choice for marine, chemical, and high-exposure industrial applications compared to other stainless grades.
Stainless steel 316L offers low carbon for sensitization resistance, 316H provides higher carbon for enhanced thermal stability, and 316Ti has titanium for advanced intergranular corrosion resistance in demanding environments.
Passivation forms a protective chromium oxide layer, improving corrosion resistance by removing free iron or impurities. Chemical treatments followed by neutralization ensure maximum durability in aggressive environments.
Stainless steel 316 is widely used in marine construction, food processing, chemical processing, pharmaceutical equipment, automotive manufacturing, and architectural components requiring high corrosion and heat resistance.
Key factors include certified quality processes, technical support, custom fabrication, inventory range, fast delivery, and documentation for industry compliance, particularly for regulated industries like food, pharma, and marine.
Leading North American suppliers include Cleveland-Cliffs, Source 21, and Aperam’s Sterling Heights, Michigan facility, all offering stainless steel 316 for industrial, construction, and marine projects.

As discussed earlier, stainless steel 316 is part of the austenitic family, where nickel acts as a stabilizer for the austenitic structure. The typical composition of stainless steel 316 includes 16–18% chromium, 10–14% nickel, 2–3% molybdenum, up to 2% manganese, up to 0.75% silicon, up to 0.10% nitrogen, up to 0.08% carbon, up to 0.045% phosphorus, up to 0.03% sulfur, with iron making up the remainder. Additional alloying elements like titanium and niobium may be included to produce other grades. The compositions of various stainless steel grades are summarized below.
| Grade | Alloying Elements (Percent) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Cr | Ni | Mo | Mn | Si | N | P | S | Others | |
| 316 | 0.08 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 | 0.045 | 0.03 | |
| 316L | 0.03 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 | 0.045 | 0.03 | |
| 316H | 0.04 - 0.10 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.045 | 0.03 | ||
| 316Ti | 0.08 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 | 0.045 | 0.03 | Ti¹ |
| 316Cb | 0.08 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 | 0.045 | 0.03 | Cb² |
| 316N | 0.03 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 - 0.16 | 0.045 | 0.03 | |
| 316LN | 0.03 | 16-18 | 10-14 | 2-3 | 2.0 | 0.75 | 0.10 - 0.16 | 0.045 | 0.03 | |
Notes:
The alloying elements of stainless steel 316 and their effects on the properties of the alloy are listed below.
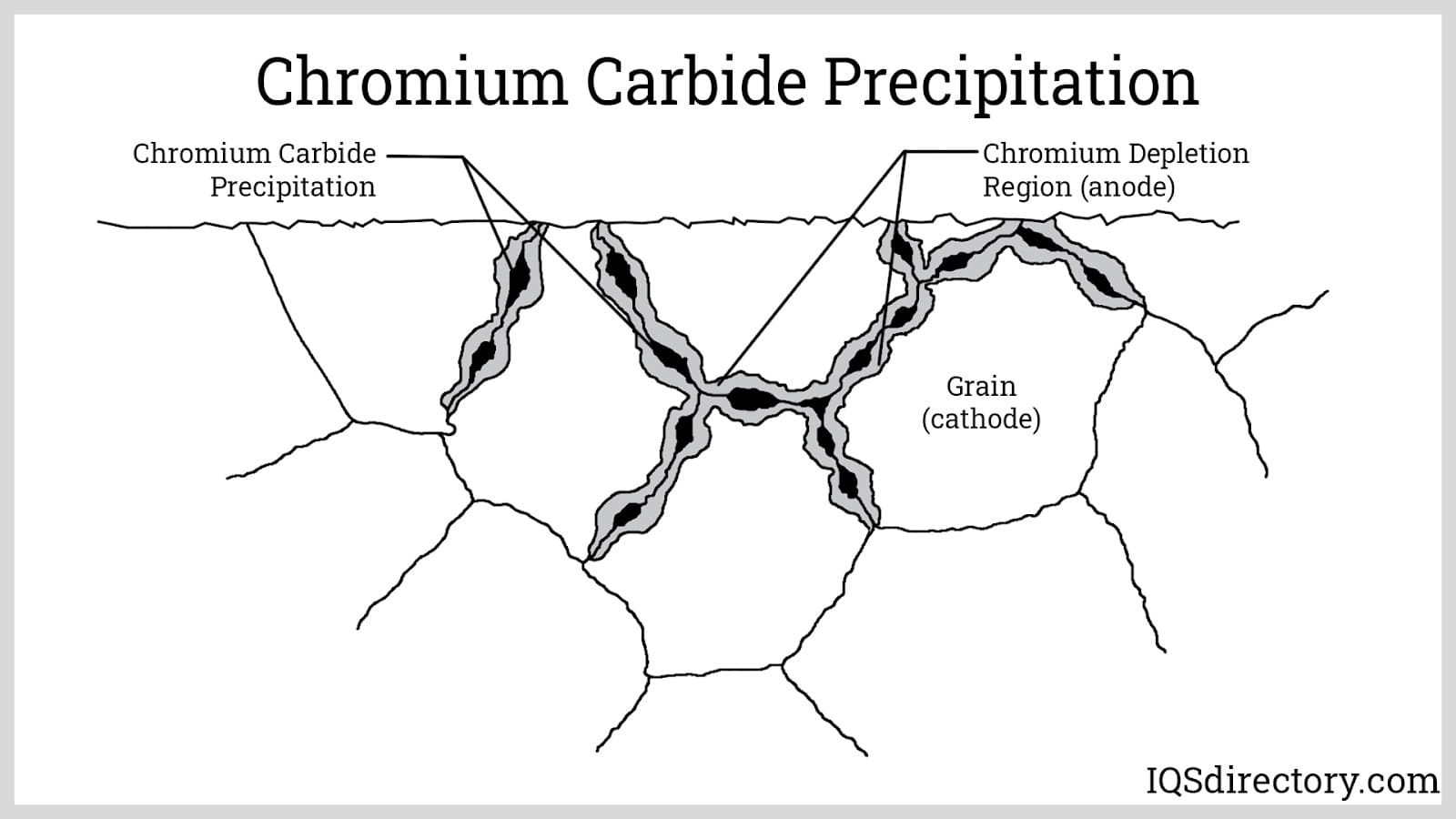
Carbon: This is the main alloying element of steel. Iron alone has poor mechanical properties, but when alloyed with varying amounts of carbon imparts a wide range of hardness and strength. Adding carbon makes the steel harder and stronger but more brittle. Decreasing it improves ductility. Also, adding sufficient amounts of carbon allows the steel to respond to heat treatment. However, there is a certain limit to how much carbon can be added. For austenitic stainless steels, adding too much carbon promotes sensitization. Sensitization is the precipitation of chromium carbides at the grain boundaries that consumes the chromium from the adjacent regions. This makes the stainless steel susceptible to intergranular corrosion.
Nickel: Nickel is added to stainless steel to form or retain an austenitic microstructure at room and low temperatures. The minimum amount required to stabilize an austenitic microstructure is around 8 to 9%. In austenitic stainless steels, 10 to 14% is required due to the addition of molybdenum, another ferrite former aside from chromium.
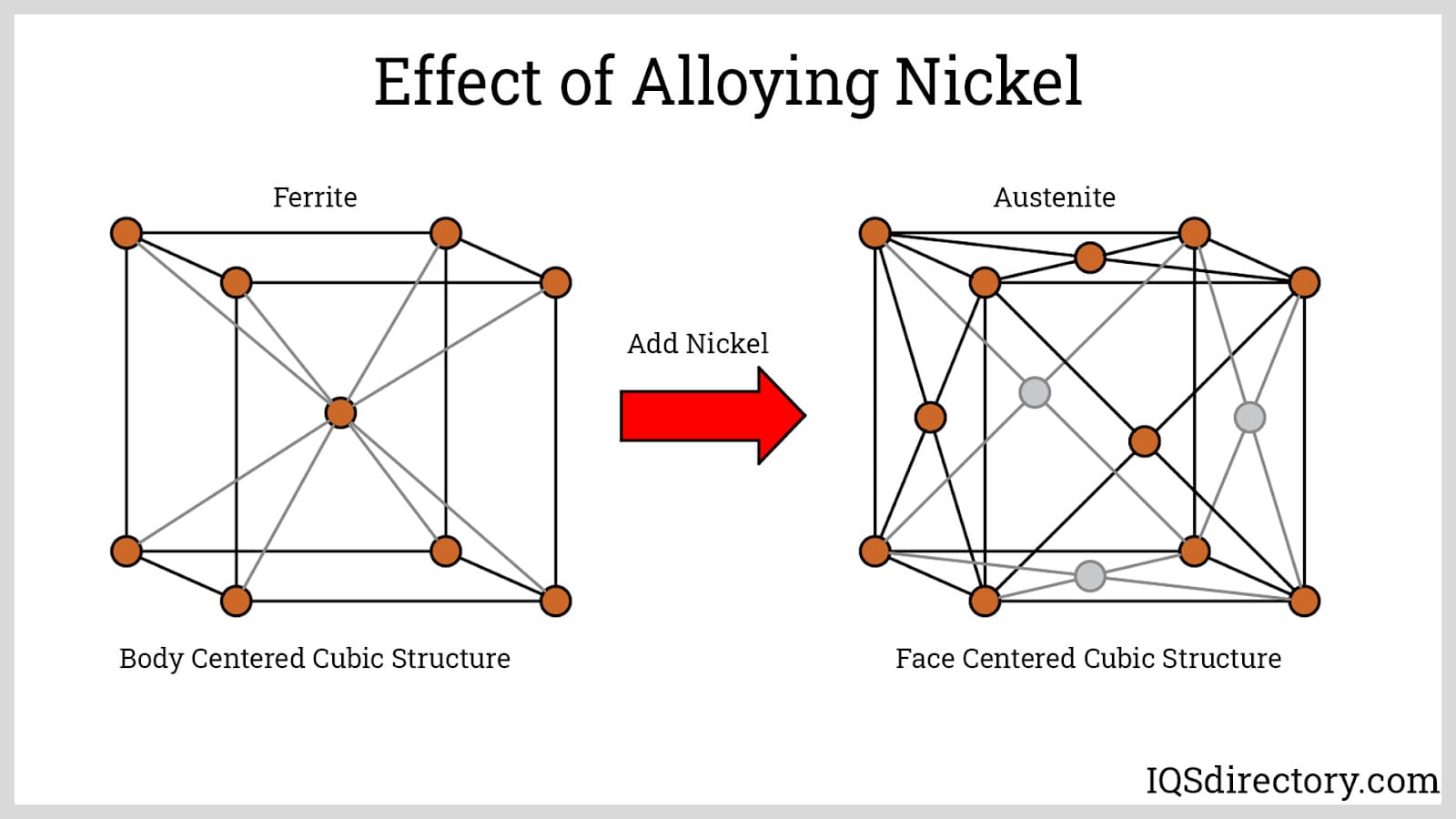
Titanium: Titanium is a stabilizer added to standard or straight 316 stainless steels to form the 316Ti variant. Titanium is a stronger carbide-former than chromium. At high temperatures, chromium tends to react with carbon and precipitate at grain boundaries. In stainless steel 316Ti, titanium reacts with carbon instead of chromium. This maintains the amount of chromium present within the austenite, resulting in the high-temperature stability of 316Ti. By lessening the formation of precipitates, intergranular corrosion resistance is improved.

Stainless steel 316 is the second most commonly used stainless steel grade after 304. It is favored for its addition of molybdenum, which enhances its resistance to chemical attacks, particularly from chloride solutions. Beyond the role of molybdenum, many of its beneficial properties are due to its austenitic microstructure.

Below is a summary of the general properties of stainless steel 316 and its variants. These properties highlight its advantages compared to other types of stainless steel.
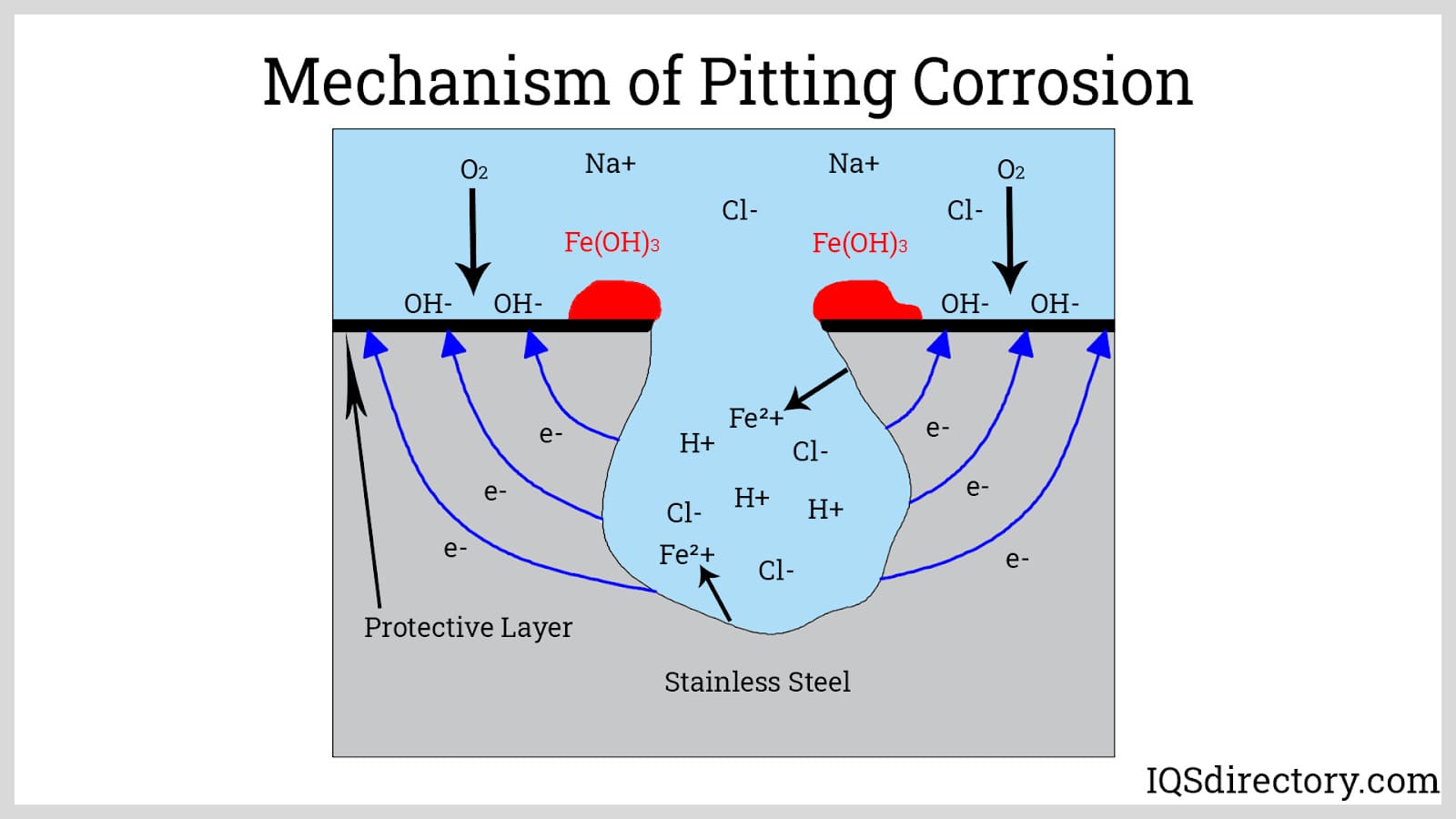
Corrosion Resistance: All stainless steel 316 grades have molybdenum as an alloying element that further improves corrosion resistance, particularly pitting corrosion. Pitting is a highly localized type of corrosion that creates shallow holes on the surface of the metal. This takes place in the presence of solutions containing chloride ions, such as seawater. High resistance to pitting corrosion makes stainless steel 316 recommended for marine applications. Molybdenum, together with chromium and nitrogen, is one of the factors in determining the pitting index or pitting resistance equivalence number.
Weldability: Austenitic stainless steels experience fewer negative effects from welding. They can retain their toughness and impact strength since they do not transform to martensite. They are less susceptible to cold cracking as encountered in martensitic stainless steels. Because of these, they are suitable for welding fillers even in welding different stainless steel groups.

Listed below are the various grades of stainless steel 316. These grades are modified versions of the standard 316 composition, where the carbon content is reduced or stabilizing alloying elements are added. These modifications enhance or maintain mechanical properties and corrosion resistance, particularly after welding. Variants with higher carbon and nitrogen contents are utilized for their improved hardness and creep resistance.

316L: As of now, this is perhaps the most widely used variant compared to the standard and the 316Ti grade. Originally, low carbon grades were more expensive and difficult to produce until the introduction of the production process known as Argon Oxygen Decarburization (AOD). This grade of stainless steel 316 has a lower carbon content to reduce the effects of sensitization.
Lower carbon content means less formation of chromium carbide precipitates and less depletion of chromium in regions near the grain boundaries. This improves the retention of toughness and corrosion resistance of the stainless steel after welding.
316H: This grade contains higher amounts of carbon, improving its thermal stability and creep resistance. Its corrosion resistance is comparable to 316L. However, due to the high carbon content, it is prone to sensitization which makes welding joints vulnerable to corrosion.


Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...

Stainless steel grades each consist of carbon, iron, 10.5%-30% chromium, nickel, molybdenum, and other alloying elements. It is a popular metal used in various products, tools, equipment, and structures that serve in many industrial, commercial, and domestic applications...

Stainless steel can be fabricated using any of the traditional forming and shaping methods. Austenitic stainless steel can be rolled, spun, deep drawn, cold forged, hot forged, or stippled using force and stress...

Stainless steel tubing is a multifaceted product that is commonly utilized in structural applications. Stainless steel tubing diameters and variations vary greatly based on the application requirements and are...

Perforated stainless steel is cut, punched, or stamped to produce a precise pattern of holes or apertures. It is used for functional reasons, such as filtration or ventilation, and aesthetic ones, such as architectural accents...

Stainless steel hinges connect two components while allowing them to move in relation to one another. They can have various leafs shaped like rectangles and other forms depending on the type of stainless steel hinge. Stainless steel hinge leafs are...

Stainless steel tanks are widely used in food, beverage, dairy, medicine, cosmetics, and other manufacturing processes where cleanliness and purity are important. These are also used in industrial plants for storing chemicals and gases where strong resistance from chemical degradation is required...

Titanium metal, with the symbol Ti, is the ninth most abundant element in the earth‘s crust. It does not occur in large deposits, yet small amounts of titanium are found in almost every rock...