Aluminized Steel

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...
Please fill out the following form to submit a Request for Quote to any of the following companies listed on
Information about titanium metals, grades, uses, and chemistry with a list of manufacturers.
You will learn more about topics such as:

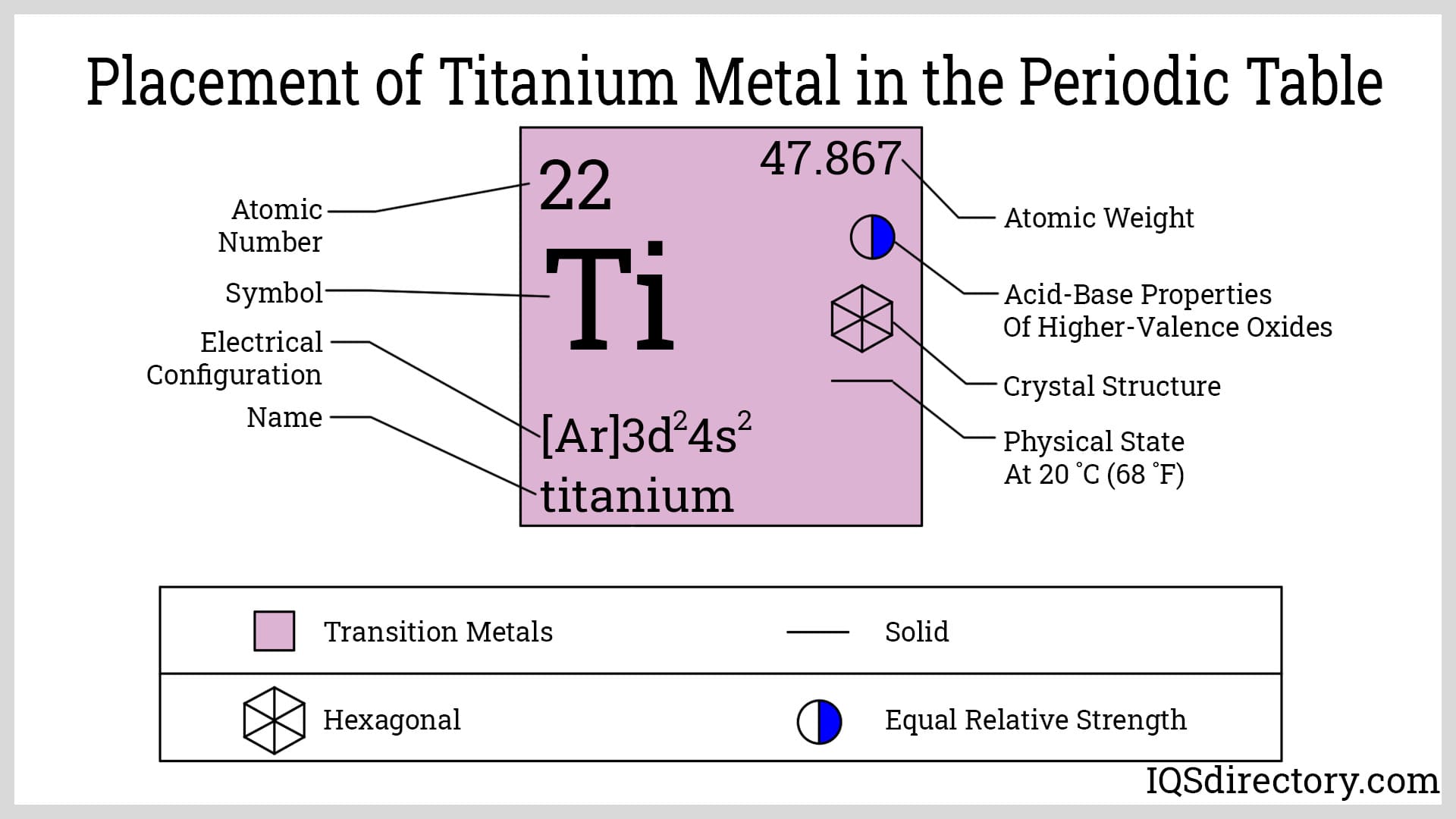
Titanium, symbolized as “Ti” and possessing the atomic number 22, is a chemical element with an atomic mass of 47.9. It stands as the ninth most plentiful element in the Earth's crust. The primary sources of titanium include minerals like rutile (titanium dioxide), ilmenite (iron titanium oxide), and sphene (calcium titanium silicate).
Esteemed for its high strength-to-weight ratio, ductility, corrosion resistance, and biocompatibility, titanium is favored in various industries, especially for medical implants. Its remarkable strength and resistance to heat, water, and salt enhance its lightweight attributes. These factors render titanium an attractive option for both everyday products such as jewelry and essential applications including medical devices, aircraft, and marine vessels.
Titanium alloys preserve the fundamental properties of pure titanium while introducing traits like flexibility and malleability, broadening their usability. Pure titanium is available in six grades—1, 2, 3, 4, 7, and 11—forming the basis for four main titanium alloys. These alloys are enhanced by elements like aluminum, molybdenum, vanadium, niobium, tantalum, zirconium, manganese, iron, chromium, cobalt, nickel, and copper. The prominent titanium alloys include Ti 6Al-4V, Ti 6Al ELI, Ti 3Al 2.5V, and Ti 5Al-2.5Sn.
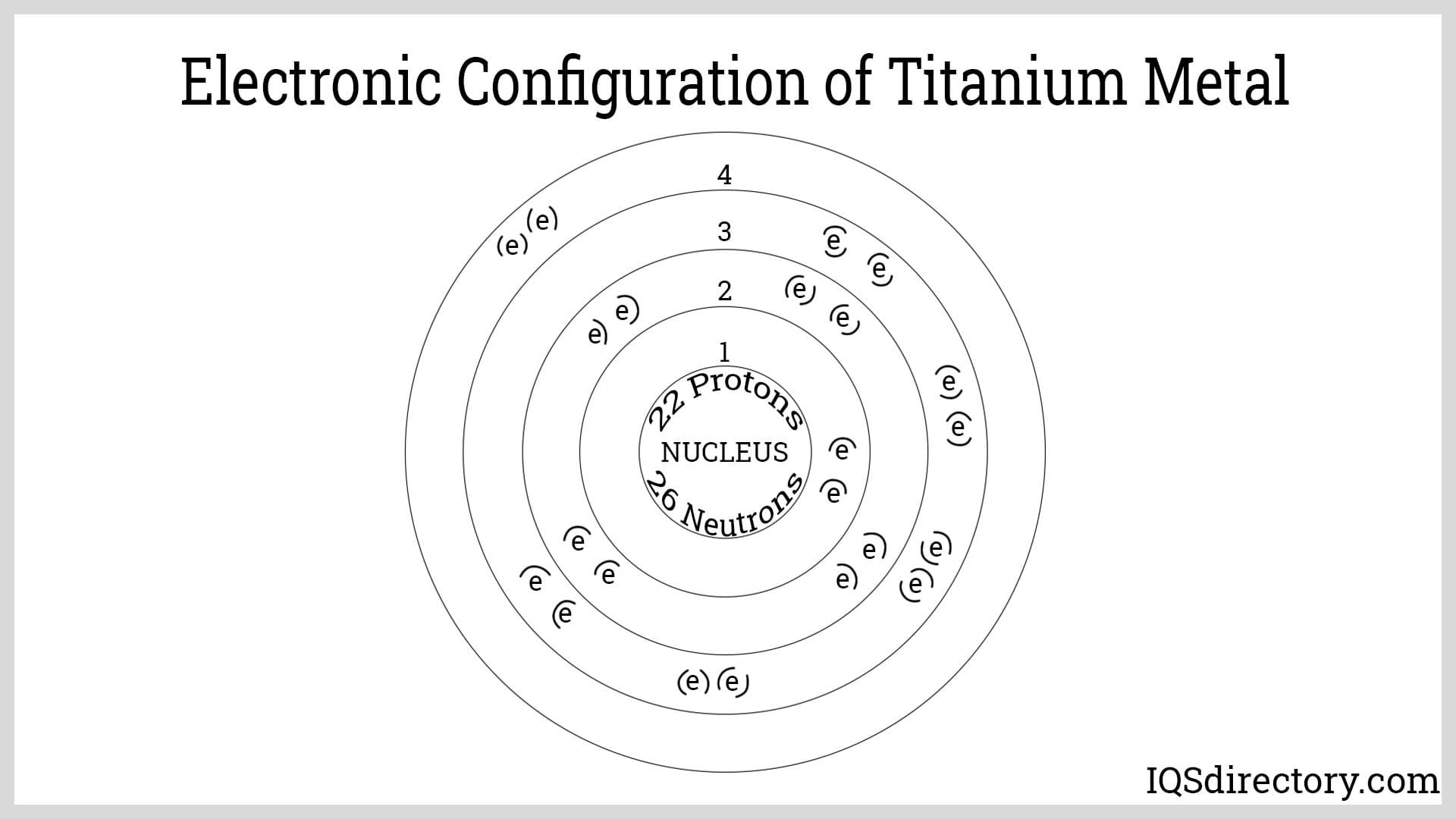
Titanium is the first element in the D-block of the periodic table. It has 22 electrons and 22 protons, placing it in period 4, group 4, defined by its electron configuration. The arrangement for titanium is 1s² 2s² 2p⁶ 3s² 3p⁶ 3d² 4s². These electrons, particularly in the fourth orbital, contribute to titanium's chemical bonding capabilities and various properties.
Titanium accounts for about 0.63% of the Earth's crust, with nearly 90% occurring in ilmenite minerals, which are comprised of iron titanium oxides (FeTiO₃). The remaining titanium is scattered among other minerals, including anatase, perovskite, rutile, leucoxene, and sphene.
The extraction of titanium involves sourcing from sand, rocks, soil, and clay using open-pit or underground mining methods, based on deposit location. To improve titanium content and eliminate impurities, techniques like crushing, grinding, screening, magnetic separation, and flotation are applied.
Among the five stable isotopes of titanium metal are titanium-46, titanium-47, titanium-48, titanium-49, and titanium-50. The most common, titanium-48, comprises 73.8% of naturally found titanium. Also, there are 21 identified radioisotopes of titanium, with the most stable being titanium-44, titanium-45, titanium-51, and titanium-52, each having unique half-lives. Titanium-44 has a half-life of 63 years, titanium-45 lasts 184.8 minutes, titanium-51 persists for 5.76 minutes, and titanium-52 has a half-life of 1.7 minutes.
The most prevalent isotope, titanium-48, makes up 73.8% of the element's natural occurrence.
Titanium-46 (46Ti) 8% abundant
Titanium-47 (47Ti) 7.8% abundant
Titanium-48 (48Ti) 73.8% abundant
Titanium-49 (49Ti) 5.5% abundant
Titanium-50 (50Ti) 5.3% abundant
Titanium typically exhibits an oxidation state of +4, which means it cannot be further oxidized in chemical reactions. As a transition metal, it can display multiple oxidation states: +2, +3, and +4. Among these, +4 and +2 are considered the most stable.
When exposed to air, titanium and its alloys quickly form a layer of titanium oxide. While titanium reacts readily with air, its reaction with water is slower due to a protective oxide coating. Initially 1 to 2 nanometers (nm) thick, this coating can grow to 25 nm over four years, providing titanium's notable corrosion resistance.
Titanium is known to have 21 radioisotopes with atomic weights ranging from 39.99 unified atomic mass units (u) for titanium-40 to 57.966 u for titanium-58, each with distinctive half-lives. The most stable among them are titanium-44, titanium-45, titanium-51, and titanium-52.
| Nuclide Symbol |
Z(p) | N(n) | Isotopic Mass (u) | Half-Life | Nuclear Spin |
| Excitation Energy | |||||
| 44Ti | 22 | 22 | 43.9596901(8) | 60 years | 0+ |
| 45Ti | 22 | 23 | 44.9581256(11) | 184.8 min | 7/2- |
| 51Ti | 22 | 29 | 50.946615(1) | 5.76 min | 3/2- |
| 52Ti | 22 | 30 | 51.946897(8) | 1.7 min | 0+ |

Titanium is an inert metal renowned in the materials science and metals industry for its exceptional physical properties. Thanks to its high strength-to-weight ratio, titanium metal is particularly valued in aerospace engineering, medical device manufacturing, automotive production, and sports equipment, all sectors that demand lightweight yet highly durable materials. This unique combination of low density (4.5 g/cm³) and remarkable strength enables titanium to outperform traditional metals such as stainless steel and aluminum in many high-performance applications, including aircraft components, prosthetics, and surgical instruments. The high melting point of titanium, exceeding 3000°F (1650°C), and its boiling point of 5948°F (3287°C), reinforce its advantageous refractory and heat-resistant qualities—making it ideal for environments with extreme thermal requirements, such as jet engines and power plants.
In an oxygen-free environment, titanium exhibits striking ductility and formability, making it practical for intricate forming processes in industrial manufacturing. Its naturally lustrous, gray-white appearance, along with excellent surface stability, makes it a popular choice for high-quality metal coatings and decorative finishes. Additionally, titanium dioxide (TiO2), a critical compound derived from titanium, is valued for its clarity, high refractive index, and ability to impart brilliant whiteness and optical dispersion—properties highly sought after in pigments, coatings, and UV-resistant materials. Unlike good conductors like copper, titanium is characterized by low thermal and electrical conductivity, which provides insulation benefits in certain engineering applications. Below its critical temperature of 0.49 K, titanium becomes superconducting, adding value to advanced research and cryogenic technologies. In its elemental form, titanium can become mildly radioactive when bombarded with deuterons, but remains safe for most commercial and industrial uses due to its general chemical inertness.

| Physical Properties of Titanium Metal | |
|---|---|
| Property Name | Description |
| Appearance | Silvery, Gray-white, Metallic |
| Strength-To-Weight Ratio | High (40%) |
| Atomic Number | 22 |
| Atomic Weight | 47.88 |
| Density At 25°C In g/cm3 | 4.5 |
| Atomic Radius In mm | 0.145 |
| Boiling Point In °C | 3287 |
| Melting Point In °C | 1668 |
| Tensile Strength In MPa | 220 |
| Modulus In GPA | 116 |
| Shear Modulus In GPA | 43.0 |
| Hardness, Brinell | 70 |
| Elongation (At Breaking Point) | 54% |
| Poisson Ratio | 0.34 |
Titanium's chemical properties closely resemble those of zirconium and silicon, as all belong to Group 4 (IVB) of the periodic table—a group noted among metallurgists and chemists for combining high melting points, corrosion resistance, and transitional metallic characteristics. Elements in this group exhibit versatility in bonding and chemical reactions, allowing them to bridge the gap between metals and nonmetals in both mineral and metallic form. In industrial settings, titanium and its alloys, such as Ti-6Al-4V, are widely recognized for their exceptional corrosion resistance and biocompatibility, making them essential in chemical reactors, seawater desalination, and biomedicine.
Titanium naturally oxidizes when exposed to air, readily forming a stable surface oxide layer at temperatures above 610°C in pure oxygen and about 1200°C in standard atmospheric conditions. This passive oxide coating confers outstanding resistance to further oxidation, protecting titanium from most corrosive agents and ensuring its longevity even in harsh chemical environments. The thickness and protective properties of this layer increase over time and with sustained exposure to oxygen-rich or aqueous atmospheres, which is why titanium is often specified in chemical processing plants, marine environments, and nuclear facilities.
User Considerations: When evaluating titanium for industrial, commercial, or medical applications, users should consider not only its mechanical and chemical resilience, but also its weldability, ease of machining, environmental compatibility, and total lifecycle cost compared to alternatives like stainless steel, nickel-based alloys, and advanced ceramics. For those intending to purchase titanium products, it’s essential to understand the specific grade (such as Grade 2, Grade 5, or medical-grade titanium), intended use (from aerospace fasteners to orthopedic implants), and the required certifications (such as ASTM, ISO, and ASME standards) to ensure optimal results and regulatory compliance. Buyers should consult leading titanium manufacturers, suppliers, and distributors for material certificates, technical support, and optimal fabrication methods to maximize both performance and cost-effectiveness.
| Chemical Properties of Titanium Metal | |
|---|---|
| Property Name | Description |
| Thermal Expansion Coefficient At 20°C - 100°C in μ/m°C | 8.90 |
| Thermal Conductivity In W/mK | 17 |
| CAS Number | 7440-32-6 |
| Thermal Neutron Cross-section | 5.6 Barns/Atom |
| Electrode Potential | 0.20 V |
| Ionic Radius | 0.680 A |
| Electronegativity (Pauling) | 1.54 |
| X-ray Absorption Edge | 2.497 A |
| Electrochemical Equivalent | 0.4468 g/A/h |
| Coefficient Of Linear Expansion At 25°C in K-1 | 8.5 x 10-6 |
| The Heat Of Transformation In kJ/mol | 3.685 |
| Latent Heat Of Fusion In kJ/mol | 20.9 |
| Latent Heat Of Sublimation In J/mol | 464.7 |
| Latent Heat Of Vaporization In kJ/mol | 397.8 |
| Specific Heat Capacity At 25°C In J g-1K-1 | 0.523 |
Titanium chemistry is primarily characterized by its +4 oxidation state, which is its most stable and prevalent form. Most titanium metal complexes exhibit an octahedral coordination geometry, except for titanium tetrachloride (TiCl₄), which possesses a tetrahedral geometry. The high oxidation state of titanium tetrachloride results in increased covalent bonding. As a transition metal, titanium also forms aqua Ti(IV) complexes (also called water ligand titanium ion complexes), which play a vital role in both industrial and biological applications. These unique coordination properties make titanium highly valuable in the synthesis of a wide variety of compounds tailored for specialized industrial, chemical, and technological uses.
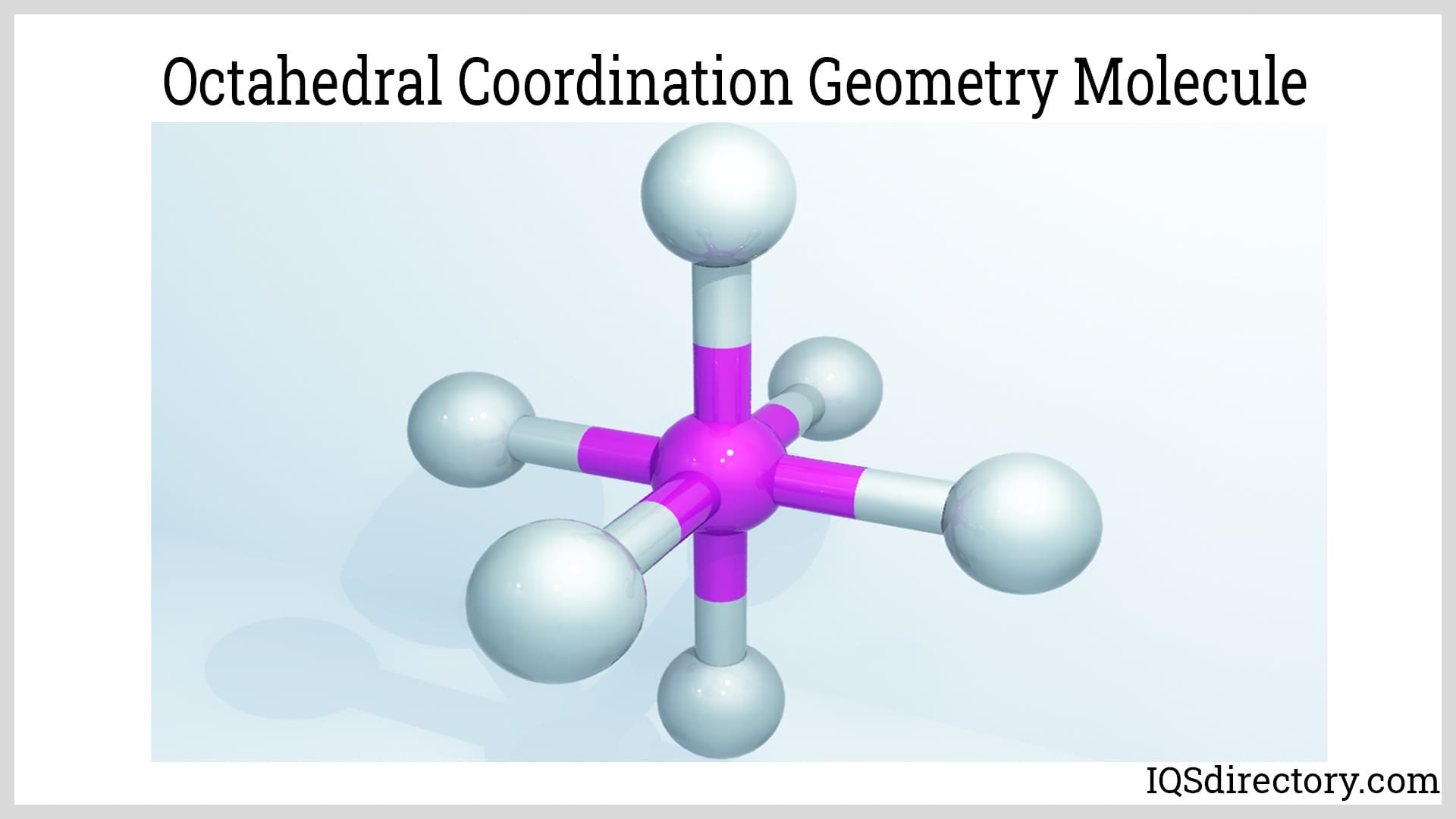
In addition, titanium oxides and sulfides exhibit remarkable stability, resistance to corrosion, and unique electronic and magnetic properties, making them indispensable not only in chemical industries but also in emerging fields like nanotechnology, green energy, and advanced materials engineering. These compounds are increasingly valued for their roles in producing eco-friendly pigments, energy-efficient coatings, and high-capacity battery electrodes.
Titanium nitrides (TiN) and titanium carbides (TiC) are part of the refractory transition metal family, renowned for their exceptional hardness, wear resistance, and stability at high temperatures. These materials are pivotal in the manufacturing of cutting tools, armor plating, and protective coatings.
Titanium nitrides exhibit properties characteristic of covalent compounds:
Titanium nitride (TiN) has a Mohs hardness of 9.0, comparable to sapphire and carborundum. TiN is widely used as a coating material for cutting tools such as drill bits, milling cutters, and medical instruments, offering both functional wear resistance and an appealing gold-like finish. Beyond its mechanical uses, TiN also functions as an essential barrier material in semiconductor device fabrication because of its chemical stability and conductivity properties. Titanium carbide (TiC), with similar mechanical and chemical properties, is frequently employed in composite ceramics and refractory linings due to its superb heat resistance and compatibility with other advanced engineering materials.
Both titanium nitrides and carbides play critical roles in modern manufacturing, electronics, and surface engineering, supporting the ongoing evolution of high-performance materials for precision, reliability, and durability.
Titanium halides are synthesized by directly reacting titanium with halogen gases (X₂). The most common of these is titanium tetrachloride (TiCl₄), a colorless and volatile liquid often observed as yellowish due to impurities in industrial settings. Upon exposure to air, TiCl₄ hydrolyzes rapidly, generating dense, white clouds—a property utilized for military smoke screens and specialized signaling applications.
Understanding the reactivity, structures, and applications of these halides is crucial for industries focused on titanium refining, chemical manufacturing, and materials engineering.
| Titanium(IV) Halides | ||||
|---|---|---|---|---|
| Formula | Color | MP | BP | Structure |
| TiF4 | White | - | 284 | Fluoride Bridged |
| TiCl4 | Colorless | -24 | 136.4 | |
| TiBr4 | Yellow | 38 | 233.5 | HCP l- But essentially monomeric cf. Snl4 |
| Til4 | Violet-Black | 155 | 377 | HCP l- But essentially monomeric cf. Snl4< |
| Titanium(IV) Halides | |||||
|---|---|---|---|---|---|
| Formula | Color | MP | BP | m (BM) | Structure |
| TiF3 | Blue | 950d | 1.75 | ||
| TiCl3 | Violet | 450d | Bil3 | ||
| TiBr3 | Violet | Bil3 | |||
| Til3 | Violet-Black | ||||
In summary, titanium compounds and alloys serve as the foundation for a vast range of high-performance materials spanning aerospace, biomedical implants, electronics, energy storage, and advanced coatings. As industries increasingly prioritize lightweight, non-corrosive, and biocompatible materials, titanium's compound versatility, physical properties, and unique chemical behavior ensure its ongoing relevance in materials development, manufacturing innovation, and sustainable technology solutions. For buyers and engineers evaluating titanium products or materials, understanding the specific properties and industrial applications of various titanium compounds is crucial for selecting the optimal form of titanium for a given use case.
Titanium stands out for its high strength-to-weight ratio, corrosion resistance, and biocompatibility. These properties make it ideal for aerospace, medical implants, marine vessels, and energy-efficient transportation components.
Titanium is mainly found in ilmenite and rutile minerals. Extraction involves open-pit or underground mining followed by crushing, grinding, magnetic separation, and flotation to increase titanium content and reduce impurities.
Titanium rapidly forms a stable oxide layer when exposed to air or water, acting as a natural barrier against corrosion—even from acids, seawater, and aggressive chemicals—ensuring longevity in chemical, marine, and industrial settings.
Titanium compounds like TiO₂, TiN, and TiCl₄ are vital in pigments, sunscreens, ceramics, catalysis, electronics, battery electrodes, and as ultra-hard coatings for cutting tools and medical devices.
The most common alloys are Ti-6Al-4V, Ti-6Al ELI, Ti-3Al-2.5V, and Ti-5Al-2.5Sn. These combine strength, flexibility, and corrosion resistance with lightness, supporting applications in aerospace, medicine, and automotive industries.
Titanium’s outstanding resistance to seawater corrosion and its ability to withstand chloride environments make it a premier choice for ships, desalination plants, and marine hardware.
The Kroll process is employed to convert crude titanium into titanium metal. This process involves several stages: extraction, purification, sponge production, alloy creation, and forming and shaping. Due to the high cost and time required for each step, no single industry typically handles all five stages. Instead, different industries specialize in various stages of the process.
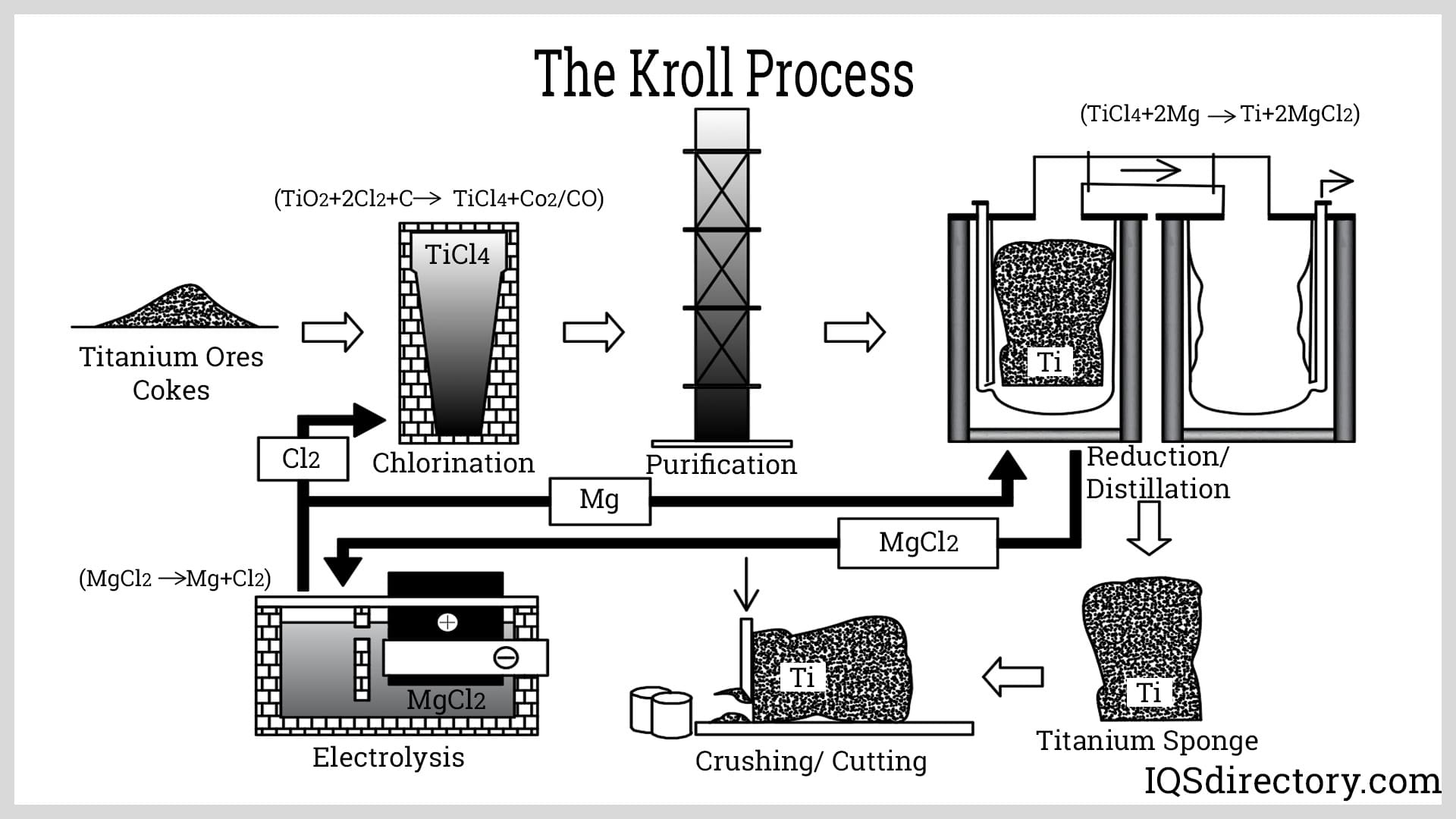
In the extraction process, ore containing titanium minerals is shipped to a processing facility. Among the various types of ore, rutile and ilmenite are the most commonly used. Ilmenite must be pre-processed to remove its iron content. The ore is placed in a fluidized bed reactor with chlorine and carbon, then heated to 900°C. This reaction produces titanium tetrachloride in an impure form, with carbon monoxide as a byproduct. To obtain titanium dioxide, impurities, including iron, must be removed from the titanium tetrachloride.
Titanium tetrachloride is purified through high-temperature vacuum distillation. The metal from the extraction process is placed into large distillation tanks and heated. During this purification process, impurities are separated via fractional distillation and precipitation. As different elements have distinct boiling points, they are removed sequentially as they reach their respective boiling points. The impurities removed include vanadium, silicon, magnesium, zirconium, and iron.
In the sponge formation stage, purified titanium tetrachloride is transferred into a stainless steel reactor vessel while still in liquid form. Magnesium is added to the vessel, and the mixture is heated to 1100°C, allowing the magnesium to react with chlorine and form magnesium chloride. Argon gas is then introduced into the vessel to displace air and prevent reactions with oxygen and nitrogen. The resulting titanium, though not pure, is in solid form. It is extracted from the vessel through a boring process and treated with a mixture of water and hydrochloric acid to remove any residual magnesium and magnesium chloride. The final product is titanium in the form of sponge.
The pure titanium sponge is blended with various alloys and scrap metals in a consumable electrode arc furnace to produce alloys. After melting and mixing the metals in the correct proportions, the mixture is compacted and welded into a sponge electrode. This electrode is then melted in a vacuum arc furnace to produce ingots, which are further processed to create a range of industrial and commercial products.
The ingots are removed from the furnace, inspected for defects, packaged, and shipped to be used to manufacture titanium alloy goods. The properties of each ingot are checked to ensure they meet customer requirements. The ingots go through different processes, such as welding, forming, casting, forging, and powder metallurgy during product manufacturing.
When titanium is purified, magnesium and magnesium chloride, byproducts of the Kroll process, are left behind. These byproducts are recycled in specialized cells where magnesium and chlorine are separated into their stable forms: solid magnesium and chlorine gas. During the recycling process, chlorine gas is captured at the top of the cell, while the solid magnesium is recovered. Both the solid magnesium and chlorine gas are saved for reuse in the Kroll process.

In the fourth stage, pure titanium sponge is combined with various alloys and scrap metals using a consumable-electrode arc furnace to produce usable alloys. Once the metals are melted and mixed in the correct proportions, the mixture is compacted and welded into a sponge electrode. This electrode is then melted in a vacuum arc furnace to produce ingots. These ingots are typically melted multiple times to ensure they meet commercial quality standards.
In the final stage of the Kroll process, ingots are removed from the furnace and inspected for defects before being sent out for further processing. Each ingot's properties are evaluated to ensure they meet customer specifications. The ingots undergo various procedures, including welding, forming, casting, forging, and powder metallurgy, to be shaped into the final product. The specific processes used depend on the requirements of the finished product.
During the Kroll process, the separation of titanium from impurities leaves behind a substantial amount of magnesium and magnesium chloride. These byproducts are promptly recycled in a specialized cell. The recycling cell separates the magnesium and chlorine into their stable forms: solid magnesium and chlorine gas. The chlorine gas is collected from the top of the cell, and both the solid magnesium and chlorine gas are reused in the Kroll process.
There are different grades of titanium, each of which is suited for specific application. Titanium CP4, or Grade 1, is the softest grade, offering the highest levels of ductility, toughness, and corrosion resistance. Its excellent cold-forming and welding properties make it widely used in architecture, automotive production, the medical field, and various processing industries. Grade 1 is offered in forms such as bars, flanges, sheets, welding wires, and forgings.
Another grade with excellent cold-forming properties, corrosion resistance, and welding capabilities is CP3, or Grade 2. This grade is utilized in a range of industries, including aerospace, automotive production, chemical processing, architecture, marine applications, and the medical field.
The following tables outline the available standards and forms for various grades of pure titanium.
| S. No. | Grade | Standards | Available Forms |
|---|---|---|---|
| 1 | CP4 – Grade 1 | ASME SB-363, ASME SB-381, ASME SB-337, ASME SB-338, ASME SB-348, ASTM F-67, ASME SB-265, ASME SB-337, ASME SB-338 | Bars, Flanges, Sheets, Welding Wires, and Forgings |
| 2 | CP3 – Grade 2 | ASME SB-363, ASME SB-381, ASME SB-337, ASME SB-338, ASME SB-348, ASTM F-67, AMS 4921, ASME SB-265, AMS 4902, ASME SB-337, ASME SB-338, AMS 4942 | Bar, Fittings, Flanges, Forgings, Pipe, Plate, Sheet, Tube, Welding Wire, Wire |
| 3 | CP2 – Grade 3 | ASME SB-363, ASME SB-381, ASME SB-337, ASME SB-338, ASME SB-348, ASTM F-67, AMS 4921, ASME SB-265, AMS 4900, ASME SB-337, ASME SB-338, AMS 4942 | Bar, Fittings, Flanges, Forgings, Pipe, Plate, Sheet, Tube, Welding Wire, Wire |
| 4 | CP1 – Grade 4 | ASME SB-363, ASME SB-381, ASME SB-337, ASME SB-348, ASTM F-67, AMS 4921, ASME SB-265, AMS 4901, ASME SB-338 | Bar, Forgings, Sheet, Welding Wire, Wire |
| 5 | Grade 7 | ASME SB-363, ASME SB-381, ASME SB-337, ASME SB-338, ASME SB-348, ASME SB-265, ASME SB-337, ASME SB-338 | Bar, Forgings, Plate, Sheet, Tube, Welding Wire, Wire |
| 6 | Grade 11 – CP Ti-0.15Pd | ASME SB-338 | Tube |
The following tables provide the standards for the different grades of titanium.
| S. No. | Grade | Standards | Available Forms |
|---|---|---|---|
| 1 | Grade 5 – Titanium 6Al-4V | ASME SB-265, AMS 4911, ASME SB-348, AMS 4928, AMS 4965, AMS 4967 | Various |
| 2 | Grade 6 – Titanium 5Al-2.5Sn | ASME SB-381, AMS 4966, MIL-T-9046, MIL-T-9047, ASME SB-348, AMS 4976, AMS 4956, ASME SB-265, AMS 4910, AMS 4926 | Bar, Forgings Plate, Sheet, Wire |
| 3 | Grade 9 – | AMS 4943, AMS 4944, ASME SB-338 | Bar, Forgings Plate, Sheet, Wire |
| 4 | Grade 12 – Ti-0.3-Mo-0.8Ni | ASME SB-338 | Tube |
| 5 | Grade 19 – Titanium Beta C | MIL-T-9046, MIL-T-9047, ASME SB-348, AMS 4957, AMS 4958, ASME SB-265 | Various |
| 6 | Grade 23 – Titanium 6Al-4V ELI | AMS 4911, AMS 4928, AMS 4930, AMS 4931, AMS 4935, AMS 4965, AMS 4967, AMS 4985, AMS 4991, MIL -T-9046, MIL-T-9047, BSTA 10,11,12, BSTA 28,56,59, DIN 3.7165, AMS 4907 ELI, AMS 4930 ELI, AMS 4956 ELI, ASTM F136 ELI, UNS R56407 | Bar, Forgings, Plate, Sheet, Welding Wire, Wire |
| 7 | 6Al-6V-2Sn – Titanium 6-6-2 | AMS 4919, AMS 4952, AMS 4975, DIN 3.7164, GE B50 TF22, GE B50TF21, GE B50TF22, GE C50TF7, MIL F-83142, MIL T-9046, MIL T-9047, PWA 1220, UNS R54620 | Bar, Plate, Sheet |
| 8 | 6Al-2Sn-4Zr-2Mo – Titanium 6-2-4-2 | AMS 4981, MIL-T-9047 | Bar, Wire Sheet, Plate, Forgings, Fittings, Flanges, Seamless Pipe, Seamless Tube, Welded Pipe, Welded Tube |
| 9 | 6Al-2Sn-4Zr-6Mo – Titanium 6-2-4-6 | AMS 4981 | Bar, Plate, Sheet |
| 10 | 8Al-1Mo-1V – Titanium 8-1-1 | MIL-T-9046, MIL-T-9047, AMS 4972, AMS 4915, AMS 4973, AMS 4955, AMS 4916 | Forgings, Bar, Sheet, Plate, Strip, Extrusions, Wire |
| 11 | Titanium 15V-3Cr- | AMS 4914, ASTM B265 | Sheet, Foil |
| 12 | 10V-2Fe-3Al | AMS 4983, AMS 4984, AMS 4986, AMS 4987 | Bar, Forgings, Plate, Sheet, Seamless Pipe, Seamless Tube, Welded Pipe, Welded Tube, Wire |
Alloy-based titanium grades, including Grades 5, 6, 9, 12, 19, and 23, are known for their excellent toughness, high strength, and strong welding and fabrication properties.
Other grades, such as Titanium 6-6-2 (6Al-6V-2Sn) and Titanium 6-2-4-2 (6Al-2Sn-4Zr-2Mo), are two-phase alpha-beta alloys that can be heat treated. These grades offer exceptional strength but exhibit lower toughness and ductility. Due to their high strength, cold forming and welding these grades can be challenging; however, they can be welded using an inert gas shield and fusion welding process. The areas affected by welding will have reduced toughness and ductility compared to the original material.
Titanium is a durable, lightweight metal with an exceptionally high strength-to-weight ratio. It is alloyed with steel to decrease grain size and with stainless steel as a carbon substitute. Its white pigmentation makes it useful in paints, paper, and plastics. The various forms of titanium are provided to satisfy the high demand for a metal that offers strength, resistance to corrosion, and resilience against fatigue and cracking.
The different types of titanium wire are pure, alloy, glasses, straight, welding, hanging, coil, bright, medical, and nickel with each type having different properties and uses. Titanium wire has all of the advantages of titanium, which makes it useful in a wide range of applications for several industries. Since its discovery in the 18th century, researchers have been developing new and innovative ways to make use of titanium wire.
Titanium wire is produced from TiO₂, Ti₃Al₂O₅, and Ti₄SiC₆, which are melted and extruded through a die or milling cutter at 1760°C (3200°F) for a brief period. This process yields wire with diameters ranging from 0.2 mm to 0.25 mm (0.008 in to 0.01 in) that can endure temperatures up to 900°C (1652°F). The high strength of titanium wire is attributed to its ductility, elasticity, and low shear strength at elevated temperatures. These properties make it especially suitable for applications in aerospace and biomedical engineering.
Similar to other wire types, titanium wire is available in a variety of gauges and grades. Grades 1 through 4 are unalloyed, while higher grades are alloyed with various metals. Titanium wire gauges range from 1 to 39, with gauge 1 being very firm, hard, and solid, and higher gauges becoming more pliable. The most commonly used gauges are between 10 and 30.

Titanium pipe is a lightweight and strong pipe that is widely used in heat exchangers. Several grades are used with grade 5 being the most used due to its distinctive strength and toughness. The strength of titanium is equal to that of steel but 57% steel’s weight. In atmospheres at 500°C (932°F), titanium pipe is able to maintain its strength and durability, which is also true at -250°C (-418°F).
Titanium pipe has a density of 4.5 g/cm³, which is 60% that of steel. Some titanium alloys offer a higher strength-to-density ratio compared to steel alloys. Furthermore, titanium pipe has a thinner wall thickness than copper while retaining its key characteristics and properties. This allows titanium pipe to be used in a wider range of applications due to its lighter weight and enhanced strength.
The strength and lightweight nature of titanium pipe make it an ideal material for airplane construction. Its durability and resistance to chemical effects also make it suitable for use in chemical processing. In the oil and gas industry, titanium pipe is valued for its ability to withstand high pressure and elevated temperatures.
Titanium pipe can be processed using four main methods: forging, rolling, extrusion, and drawing. Forging is the most commonly used method due to its ability to refine the structure of titanium. Among the remaining methods, rolling is the most widely used and is a standard process for manufacturing pipes.
Titanium flanges are employed to join titanium pipes and divide pipe networks for inspection and cleaning. Grade 2 titanium is commonly used because of its low density, which is advantageous in weight-sensitive applications. Its corrosion resistance and strength also make it well-suited for marine environments, chemical processing, and desalination systems.
Titanium flanges are designed with protruding rims that are either welded or bolted to create a secure connection in piping systems. Their primary function is to distribute the load and reinforce the connection. Weld neck titanium flanges feature a tapered end that is butt welded to the end of the pipe fitting. These flanges are resistant to deformation, making them ideal for high-pressure piping systems under various conditions.
Another type of titanium flange is the slip flange, which slides over the end of a pipe and is welded both inside and outside. It features a low hub and offers excellent strength, making it suitable for low-pressure applications.
Unlike other types of titanium flanges that are welded, threaded titanium flanges are designed for specific applications and do not require welding. They are typically used with small-diameter piping under high pressure. Threading is feasible only for smaller flanges because larger flanges are too hard to thread effectively.
The three types of titanium flanges described above are just a few of the many available. Other types include blind, integral, orifice, socket weld, plate, and custom flanges. These flanges come in various facing types such as flat, raised, ring joint, tongue and groove, and male and female. Each type is classified by its pressure-temperature rating, which indicates the maximum allowable working pressure. As the rating increases, the flange can withstand higher pressures.

Titanium plate is utilized across various industries, including medicine, due to its biocompatibility. It is available in thicknesses ranging from 2 mm (0.079 in) to 150 mm (6 in) and comes in all grades of titanium. The Kroll method is employed to produce titanium from titanium tetrachloride. Known for its toughness and exceptional strength, titanium plate effectively withstands repeated load stress.
The Kroll process for producing titanium plate is a pyrometallurgical method that starts with titanium tetrachloride, which is generated by oxidizing TiO₂ with chlorine at 1000°C (1832°F). Metal chloride impurities are distilled out to obtain a pure TiCl₄ mixture, which is then mixed with magnesium. At temperatures around 1000°C, a reaction takes place in an argon atmosphere, gradually removing the chloride to produce pure titanium in sponge form.
The sponge titanium undergoes leaching or vacuum distillation to eliminate impurities. The purified material is then jack hammered, crushed, pressed, and melted. Titanium plates are subsequently formed into various sizes and dimensions through processes such as sintering, hot rolling, and cold rolling. Each method starts with a slab of titanium that is shaped and processed to meet the desired specifications.
Titanium rods are available in various shapes, including square, hexagonal, and flat, which facilitate their storage and transport. These rods are produced through methods such as forging, rolling, extrusion, casting, and spinning. While they can serve as structural supports, titanium rods are typically melted down to manufacture other titanium products.
Titanium rods come in various shapes and are made from either pure titanium or its alloys. They possess all the advantageous properties of titanium, making them a popular choice across many industries. Common manufacturing processes for titanium rods include hot forging, hot rolling, extrusion, casting, and spinning. These techniques may be used individually or in combination, depending on the specific requirements for the titanium rod.
Titanium rods are utilized across various industrial, medical, and commercial applications, similar to other forms of titanium. In orthopedic surgery, specific types of titanium rods are used, featuring various end styles to meet patient needs. Often, titanium alloy rods are preferred for their superior strength.
Titanium rods are widely used in the aerospace industry because of titanium's lightweight and exceptional strength. Thin titanium rods are also employed in the production of notebook computers for their aesthetic appeal. Additionally, titanium finds a unique application in military armor, where it is used in armored vests that are lightweight and comfortable to wear.
Similar to titanium plates, titanium sheets are highly valued for their exceptional properties. The remarkable strength of titanium makes these sheets ideal for applications that require a lightweight yet robust metal for protection. Additionally, titanium’s non-magnetic and biocompatible qualities make it suitable for medical implants and aerospace equipment. Titanium sheets come in various grades to meet specific application needs, with Grade 2 and Grade 5 being the most commonly used, while other grades are selected for particular requirements.
Titanium sheets are manufactured similarly to titanium plates, with the main difference being their thickness. Their flexibility makes them suitable for a variety of applications. Titanium sheets are often used as heat barriers to block and prevent the spread of heat, a property that has made them a popular choice for protective materials in race cars.
Titanium sheets' resistance to heat necessitates using cold cutting methods to avoid altering their chemical properties. Common techniques for cutting and shaping titanium sheets include waterjet cutting and stamping. These methods are employed in manufacturing various titanium products, such as jet engines, springs, and deep-sea production risers.
Titanium is widely used in high-quality tubing due to its strength, low density, and excellent corrosion resistance. It serves as a lightweight alternative to steel and stainless steel. Titanium tubing has a density that is 60% lower than steel or nickel-based alloys, while offering greater strength than austenitic or ferritic stainless steels. Although stainless steel is known for its corrosion resistance, titanium surpasses it in this regard and has a higher melting point, making it a superior choice for many applications.
The various sizes of titanium tubing are used in applications that require a strong, tough, and highly resistant metal. Many of these conditions are where stainless steel fails and does not have sufficient strength. This aspect of titanium has made it one of the most dependable metals on the market.
Titanium tubing, known for its lightweight and strength, is ideal for applications where weight is a critical factor. Despite having thinner walls, titanium tubing maintains its strength and durability. It retains its stiffness and high melting point even after being shaped into various configurations. Industries and applications that rely on titanium tubing include aircraft hydraulic systems, medical implants, offshore drilling rig components, subsea equipment, and marine and chemical processing plants.
Titanium bars are produced from titanium sponge that is melted with alloying elements and then remelted through die casting or vacuum arc remelting (VAR) to form slabs. These slabs are then cast or forged to create titanium bars. Like other forms of titanium, titanium bars are lightweight, corrosion-resistant, and highly durable. They are used as raw material for various applications, including aerospace components, military armor, and architectural elements.
Raw or crude titanium is extracted from rutile, ilmenite, and sphene ores and processed using the Kroll method to produce titanium sponge. Titanium bars come in various grades, each with distinct strengths, resistance properties, and durability. The most commonly used grades for titanium bars are Grade 2 and Grade 5. Grade 2, composed of 99% titanium, is known for its low density and lightweight nature. Grade 5, alloyed with aluminum and vanadium, has a superior strength-to-weight ratio and enhanced scratch resistance compared to Grade 2.
Titanium bars are one of the most frequently sold and shipped forms of titanium by producers. They are available in various diameters, up to 35.56 cm (14 inches), and come in multiple shapes including round, square, rectangular, octagonal, and hexagonal. The specific shape of each bar is determined by the manufacturing process and the preferences of the manufacturer.
Titanium foil is a thin, highly flexible, durable, and strong layer of pure titanium with thicknesses of 0.254 mm (0.01 in) up to 0.0762 mm (0.003 in). It is formed by being rolled or pressed multiple times in order to thin it to the desired thickness. Titanium foil’s thickness gives it several advantages in regard to manufacturing and processing. Since it comes in rolled coils, titanium foil can be cut, pressed, shaped, configured, and engineered to meet the needs of a wide range of products.
Like many other forms of titanium, titanium foil starts as titanium sponge, which is melted, pressed, rolled, and cut to precise dimensions. Unlike titanium sheets, titanium foil is distinguished by its thickness, which is measured in fractions of an inch or millimeter. Titanium foil can be produced from any grade of titanium, with Grades 1, 2, 3, and 4, which are 99% pure titanium, and Grade 9 being the most commonly used.
Titanium foil is widely used because of its superior strength compared to aluminum and copper foils. It offers excellent durability in chemical environments and withstands high temperatures effectively. Additionally, titanium foil is easily molded and shaped, making it ideal for enhancing the protection of products, equipment, and devices.
Titanium is highly regarded for its strength and durability, making it ideal for applications that demand a robust metal capable of withstanding significant stress and pressure. It is lighter than steel yet as strong, and it boasts twice the strength of aluminum. With a melting point of 1,668°C (3,034°F), titanium is resistant to abrasion, cavitation, and erosion.
Most titanium ore is processed into titanium dioxide (TiO₂), and its cost can be a good indicator of titanium prices. The price of titanium dioxide fluctuates based on market conditions. Over the years, the cost of titanium metal has steadily decreased. For instance, in 2005, a metric ton of titanium was priced at $21,000, whereas by 2016, it had dropped to $3,750 per metric ton. This reduction in cost is attributed to advancements in techniques for separating oxygen atoms from pure titanium.
Compared to other metals, titanium is generally more expensive. For instance, stainless steel typically costs between $1 and $1.50 per kilogram, while aluminum ranges from $2 to $2.50 per kilogram. In contrast, titanium costs between $35 and $50 per kilogram. This significant price difference often leads manufacturers to seek alternative materials to reduce costs.
Titanium's cost-effectiveness stems from its remarkable durability and superior properties, which surpass those of other metals. Although using titanium may increase the initial cost of a product, it guarantees extended longevity and outstanding performance.
USTi offers a diverse array of titanium products, including bars, rods, pipes, tubes, plates, and sheets. As a leading metals producer, USTi processes titanium and titanium alloys, as well as zirconium, tantalum, and niobium, among others. The company serves various industries, including construction, aerospace, medicine, automotive, and marine. USTi handles all grades of titanium, from the malleable GR1 to WPT12, unalloyed titanium. Committed to customer satisfaction, USTi collaborates closely with clients to create products tailored to their specific needs and requirements.
AEE specializes in supplying high-purity metals, powders, and compounds. They provide top-quality titanium powder, ideal for producing complex parts that demand a high strength-to-weight ratio, as well as for paints, coatings, and electronic applications. AEE’s titanium powder is available in various particle sizes and spherical forms. Their mission is to deliver titanium and titanium powders with exceptional purity to meet the diverse needs of their industrial clients.
A-1 Alloys is renowned for its exceptional customer service and extensive selection of metal products, including titanium, steel, stainless steel, lead, and nickel. To cater to the specific requirements of each project or application, A-1 Alloys offers custom metal forming and shaping services. With extensive expertise in metal forming, casting, extrusion, and finishing, A-1 Alloys delivers titanium and other metals with precise tolerances and the high-quality standards the company is known for.
Reliable Source offers a diverse selection of metals tailored to fit any company's budget. Their primary objective is to reduce customer costs while delivering exceptional service, ensuring that each customer receives top-quality titanium at the most competitive prices. Reliable Source provides a comprehensive range of metal forming services, including welding, cutting, heat treating, stamping, and more. Committed to customer satisfaction, the company continually strives to advance its business with a focus on meeting customer needs.
Admat specializes in supplying titanium sheets and plates with precise thicknesses and widths tailored to specific applications. Our flat-rolled titanium can be custom-cut to lengths of up to 3000 mm (118 in) and widths of up to 1000 mm (39.3 in). We offer titanium plates in thicknesses up to 35 mm (1.38 in) and diameters up to 1000 mm (39.3 in). All products are manufactured to meet ASTM or AMS standards, with custom options available upon request.
Titanium is crucial in the medical field due to its biocompatibility. As a non-toxic material, it is widely used in surgical instruments and implants. From hip replacements to dental implants, titanium's applications are diverse and reliable. These implants can remain effective for over 20 years. Typically, titanium implants include approximately 4% vanadium and 4% to 6% aluminum.
Titanium’s unique ability to osseointegrate makes it ideal for dental and orthopedic implants, with a lifespan of up to 30 years. Its lower modulus of elasticity ensures that the load is evenly distributed between the bone and the implant, helping to reduce bone degradation and the risk of periprosthetic fractures. While titanium is stiffer than human bone, this increased stiffness can lead to bone deterioration under excessive load.
Titanium is processed into titanium dioxide, a white pigment commonly used in products like paper, toothpaste, plastics, and paints. When included in paint, titanium dioxide enhances the mixture's performance in extreme temperatures and humid conditions. Additionally, titanium dioxide is incorporated into graphite composite fishing rods and golf clubs to boost their strength.
Titanium dioxide is a chemically stable substance that resists corrosion and remains unaffected by sunlight. Its opaque quality makes it ideal as a pigment in household plastics. Additionally, titanium dioxide is utilized in sunscreens because of its high refractive index and optical dispersion properties.
Titanium is an ideal material for the manufacture of aircraft, missiles, and armor plating and is used in the manufacture of structural parts, landing gear, exhaust ducts, firewalls, and hydraulic systems. It accounts for almost 50% of materials used in aircraft production. The titanium alloys used consist of aluminum, nickel zirconium, and vanadium.

Titanium's durability and biological inertness have made it increasingly popular in the jewelry industry. Its hypoallergenic properties are especially appealing to those with sensitivities or living in humid environments. Additionally, titanium's resistance to dents, lightweight nature, and corrosion resistance make it an ideal material for crafting wristwatches and watch cases.
Artists often use titanium to craft sculptures and decorations. When combined with gold to create a 24-karat gold alloy, this blend results in an alloy that is tougher than pure 24-karat gold. Additionally, anodized titanium features optical interference fringes and a spectrum of vibrant colors, making it a popular choice for body piercings.
Titanium, known for its exceptional corrosion resistance, is highly valued in the marine industry. It is commonly used in the construction of naval ship hulls due to its ability to withstand seawater corrosion. The material is also utilized in a range of marine applications, including propeller shafts, heat exchangers, rigging, saltwater aquarium chillers, driver knives, finishing lines, and leaders. Furthermore, titanium is employed in housing and equipment for ocean-based surveillance and monitoring.
Titanium is employed in the automotive industry, especially in applications demanding both lightweight and high strength. Despite its high cost, titanium is considered cost-effective due to its superior properties compared to other metals. Its heat resistance and strength make it ideal for manufacturing exhaust and intake valves in engines.
Titanium cannot be found naturally in its metallic state. In urban environments, its concentration in the air is typically below 0.1 µg/m³. However, in areas near factories, levels can reach up to 1.0 µg/m³. This presence of titanium can impact drinking water and food. The reported daily intake of titanium by humans ranges from 300 µg to 2 mg.
Research on both animals and humans indicates that inhaled titanium dioxide remains inert. Fibrosis linked to titanium exposure is more likely due to other elements found in titanium dust rather than titanium dioxide itself. In animals, titanium nitrides, titanium hydrides, and titanium carbides have been shown to cause fibrogenic effects as well as kidney and liver dystrophy.
Titanium tetrachloride affects humans and animals differently. In humans, it can cause skin burns and eye irritation. When administered in powdered form to rats, it has been linked to the development of lymphosarcoma and fibrosarcoma. However, there is no evidence suggesting that titanium is carcinogenic to humans. Additionally, research has not demonstrated any harmful effects of titanium dioxide on the lungs.
Titanium implants and prosthetics are inert and do not affect human tissue, as both bone and soft tissue are generally tolerant of titanium. Various compounds, including dioxides, oxides, tannates, and salicylates, are significant in dermatology and cosmetics. However, exposure to specific titanium compounds has been associated with pulmonary fibrosis.

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...
Aluminum 1100 is the softest of the aluminum alloys, which makes it easy to shape and form into a wide range of products for industrial and home use. It can be cold and hot worked but is frequently shaped by...

The term "aluminum coil" describes aluminum that has been flattened into sheets where their width is significantly higher than their thickness and then "coiled" into a roll. Stacks of individual aluminum sheets are difficult to...
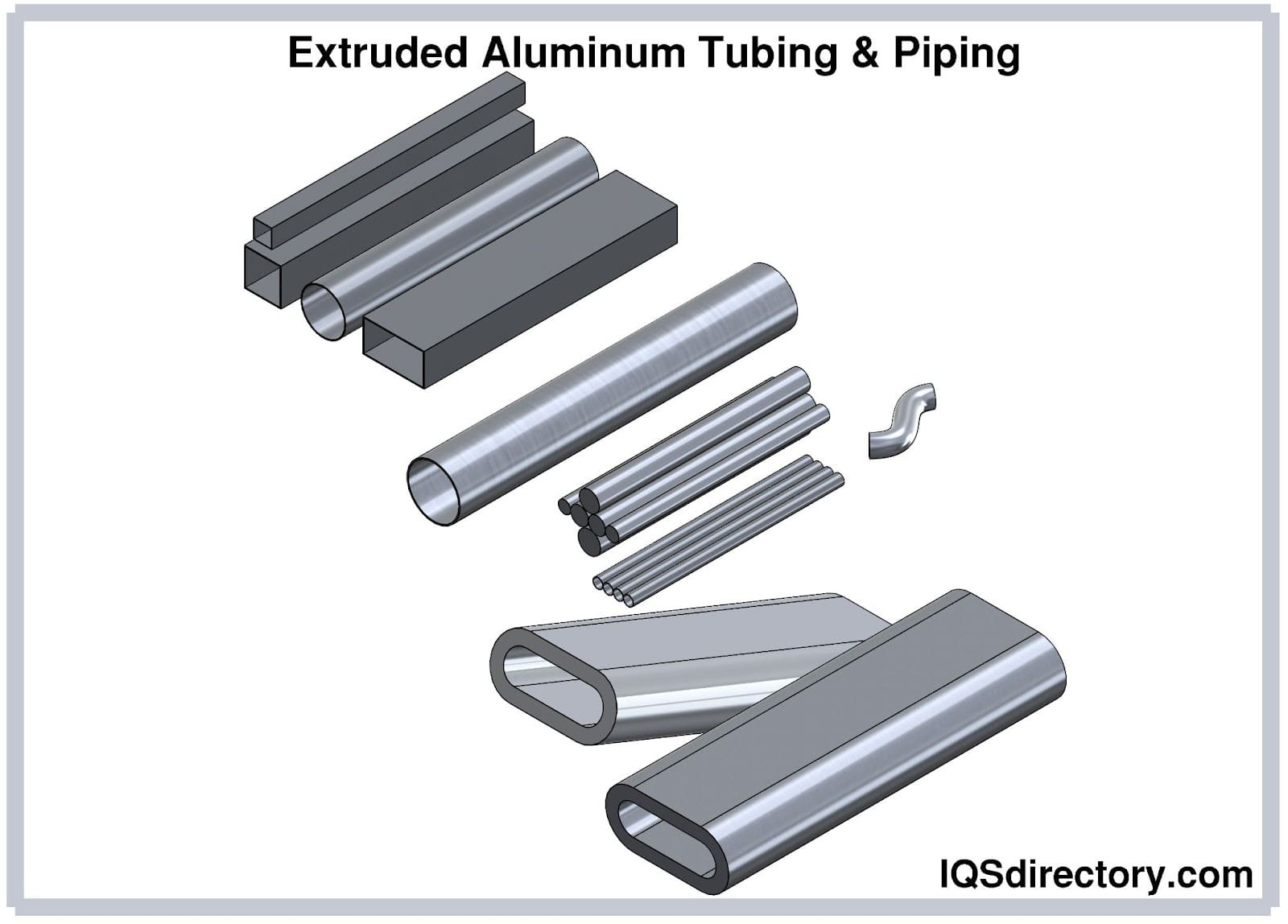
Aluminum piping and tubing is silvery-white, soft, and ductile. The metal belongs to the boron group. Aluminum is the third most abundant element present on earth. Aluminum has low density. When exposed...
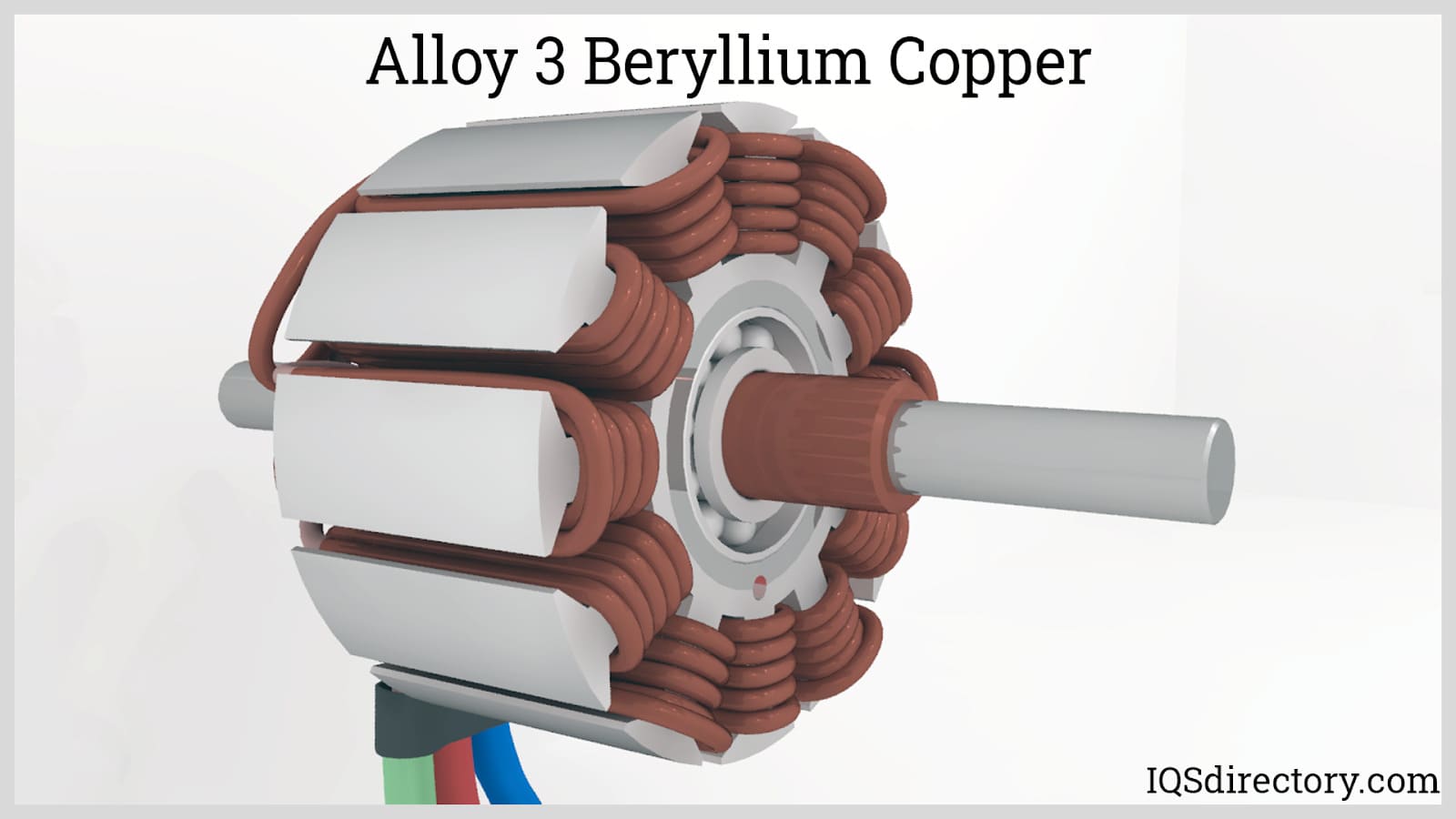
Beryllium Copper is a versatile copper alloy that is valued for its high strength and hardness, combined with good electrical and thermal conductivity. It is a non-ferrous, non-magnetic, and non-sparking metal alloy...

A variety of copper-zinc alloys are referred to together as brass. Different ratios of brass and zinc can be used to create alloys, which produce materials with various mechanical, corrosion, and thermal properties...
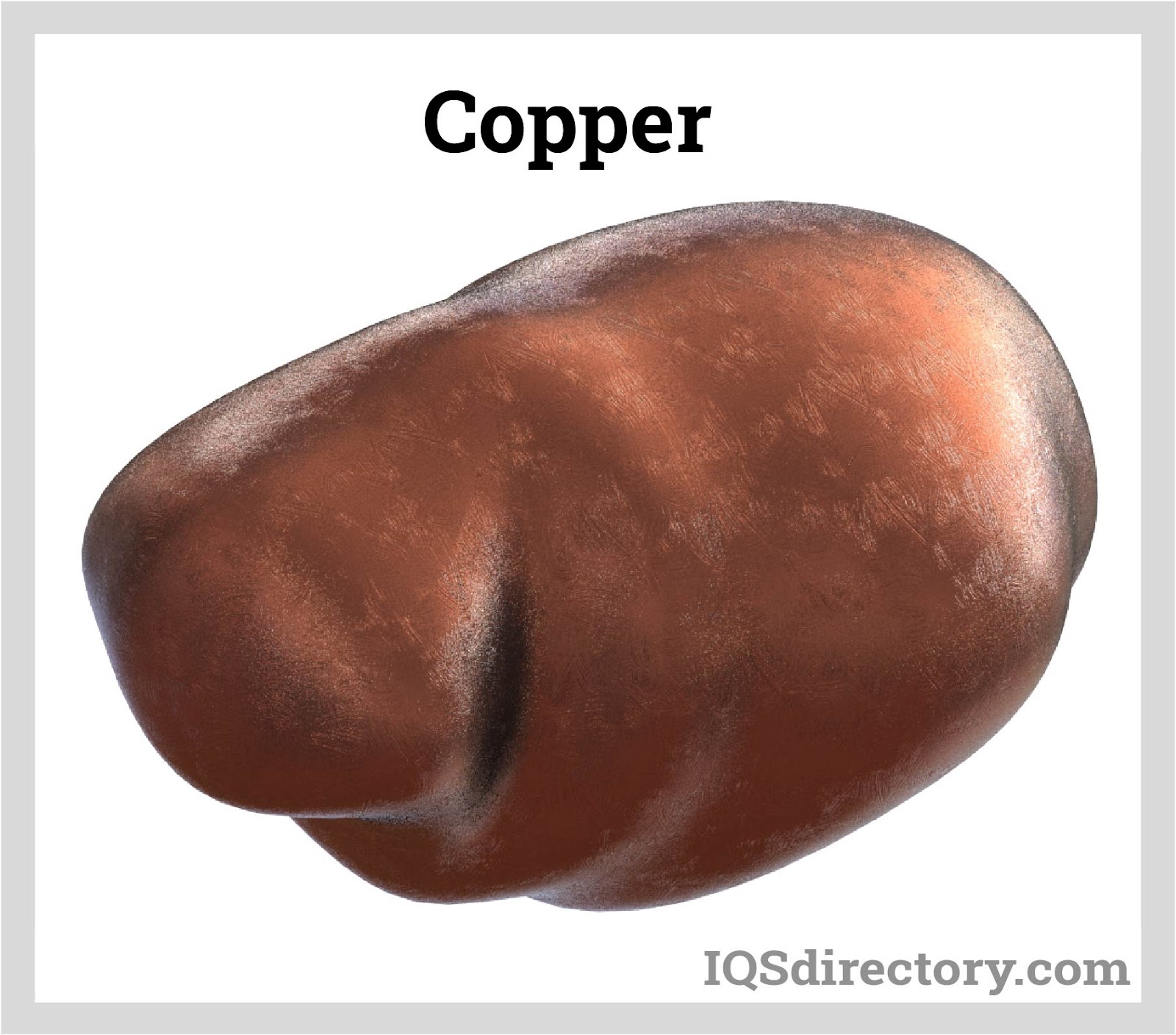
Copper is a ductile, malleable, and reddish-gold metal with the capacity to effectively conduct heat and electricity. Brass and bronze, two commonly used alloys, are created when copper is combined with...

The copper sheet is a highly malleable and workable metal with outstanding electrical and thermal conductivity and corrosion resistance. Copper (Cu) is a reddish, very ductile metal that belongs to Group 11 of the periodic table...

Metals are a group of substances that are malleable, ductile, and have high heat and electrical conductivity. They can be grouped into five categories with nickel falling in the category known as transition metals...

Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...

Stainless steel is a type of steel alloy containing a minimum of 10.5% chromium. Chromium imparts corrosion resistance to the metal. Corrosion resistance is achieved by creating a thin film of metal...

Stainless steel grades each consist of carbon, iron, 10.5%-30% chromium, nickel, molybdenum, and other alloying elements. It is a popular metal used in various products, tools, equipment, and structures that serve in many industrial, commercial, and domestic applications...

Steel service centers are companies that specialize in procuring steel directly from mills and manufacturers and supplying them to the customers. They are fundamental to the steel supply chain...

Stainless steel can be fabricated using any of the traditional forming and shaping methods. Austenitic stainless steel can be rolled, spun, deep drawn, cold forged, hot forged, or stippled using force and stress...

Stainless steel tubing is a multifaceted product that is commonly utilized in structural applications. Stainless steel tubing diameters and variations vary greatly based on the application requirements and are...

Tungsten is a rare naturally occurring chemical element on earth. It is known to be one of the toughest metals on the earth. It is usually a tin white or a steel gray metal. Tungsten is common for its high tensile...
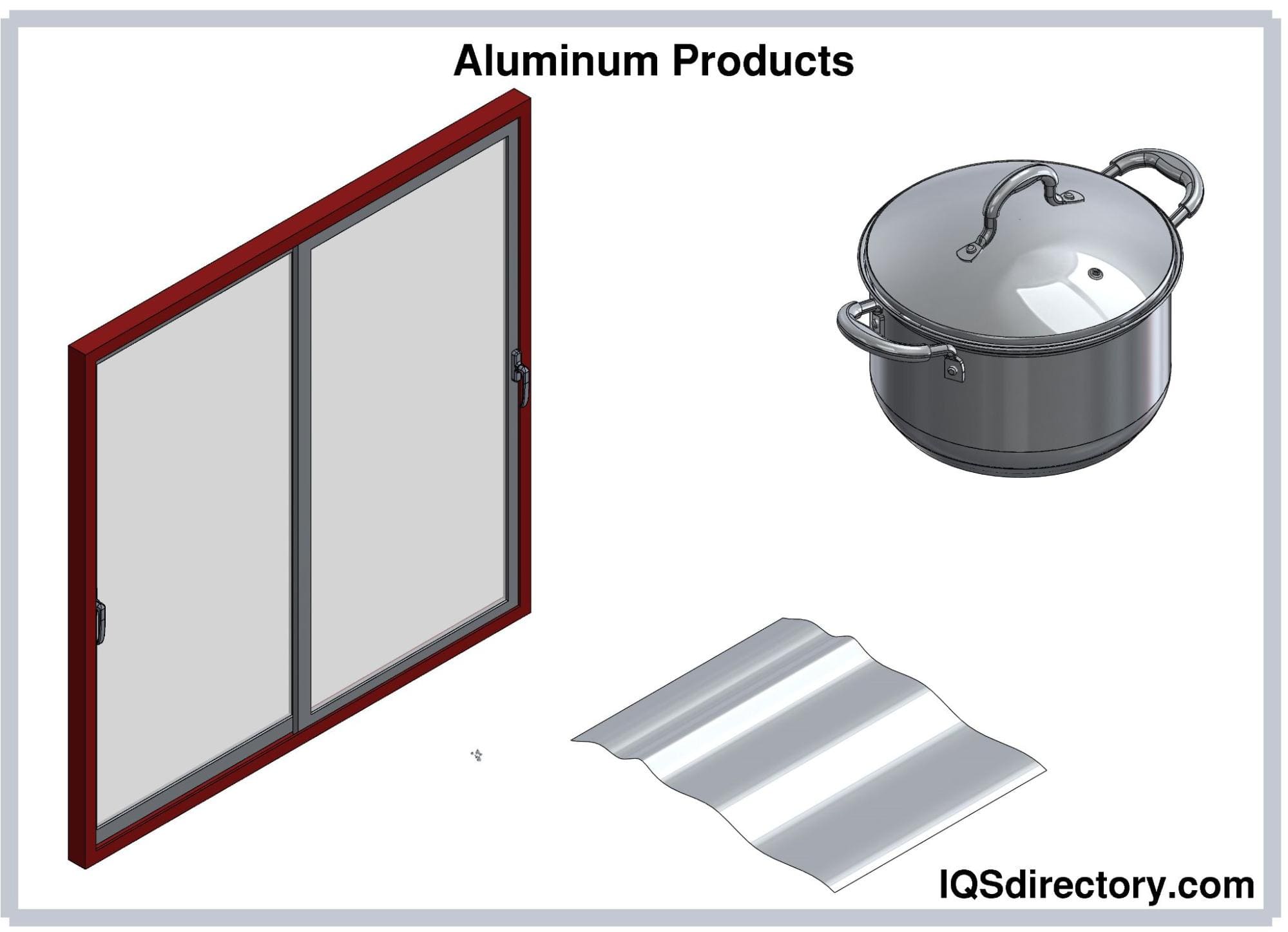
Aluminum is the most abundant metal on the Earth’s crust, but it rarely exists as an elemental form. Aluminum and its alloys are valued because of their low density and high strength-to-weight ratio, durability, and corrosion resistance...