Alloys
Alloys are a combination of two or more metals or a metal and a non-metal
that possess unique and enhanced properties compared to their individual
components. The creation of alloys dates back to the Bronze Age, where
people discovered that combining copper with other metals, such as tin,
lead, or zinc, can produce a metal with superior strength and durability.
Creating Alloys
There are several methods of creating alloys, including melting, powder
metallurgy, and solid-state reactions. Melting is the most common method,
where two or more metals are melted together at high temperatures to form a
homogeneous mixture. In powder metallurgy, metal powders are mixed and
compacted under high pressure before being heated to form a solid alloy.
Solid-state reactions involve heating two or more metals in a vacuum or
inert gas to induce diffusion and bonding between the atoms.
The purity of the constituent metals is crucial in the alloy-making process.
Any impurities can significantly affect the properties of the alloy, such as
its strength, ductility, and corrosion resistance. Therefore, manufacturers
use advanced techniques to ensure the purity of the constituent metals.
Popular Alloys
There are many alloys used in various industries, but some of the most
popular include:
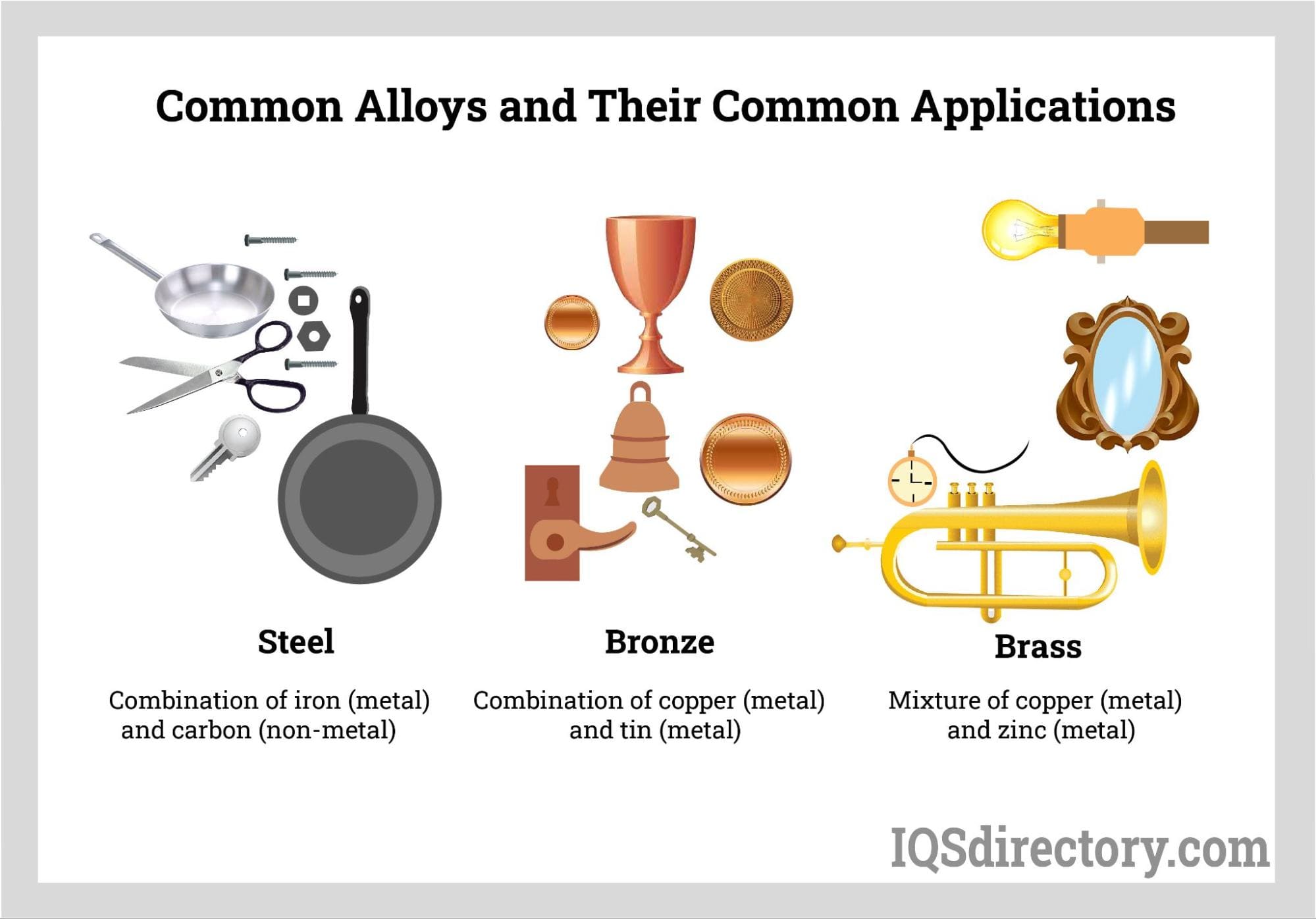
Steel
A combination of iron and carbon with small amounts of other elements, such
as manganese, silicon, and chromium. Steel is known for its high strength,
durability, and corrosion resistance.
Brass
A mixture of copper and zinc that is malleable, ductile, and
corrosion-resistant. Brass is commonly used in plumbing fixtures, musical
instruments, and decorative items.
Bronze
An alloy of copper and tin that is hard, strong, and corrosion-resistant.
Bronze is used in statues, coins, and other decorative items.
Stainless Steel
A combination of iron, chromium, and nickel that is highly resistant to
corrosion and staining. Stainless steel is used in kitchen appliances,
medical equipment, and other applications where hygiene is critical.
Aluminum Alloys
Combinations of aluminum with other metals, such as copper, magnesium, and
zinc. Aluminum alloys are lightweight, strong, and corrosion-resistant,
making them suitable for aerospace and automotive applications.
Limitations of Alloys
While alloys offer many benefits, there are also some limitations and
negative impacts associated with their use. For instance, the production of
alloys can be expensive and energy-intensive, leading to a significant
carbon footprint. Some alloys may also lack ductility, making them brittle
and prone to cracking under stress.
Benefits of Alloys
Despite their limitations, alloys provide significant benefits in various
applications. For instance, alloys can be customized to have specific
properties, such as strength, ductility, and corrosion resistance, depending
on the intended use. This makes alloys suitable for a wide range of
applications, from construction to medicine. Additionally, alloys are highly
resistant to wear and corrosion, making them long-lasting and durable, even
in harsh environments.
Applications of Alloys
Alloys can be customized to suit specific requirements by adjusting the
proportions of the constituent metals, making them a versatile material for
a wide range of applications. As a result, alloys find use in numerous
industries, such as:
Aerospace Industry
Alloys are widely used in the aerospace industry due to their high
strength-to-weight ratio and resistance to corrosion. Titanium alloys,
nickel alloys, and aluminum alloys are commonly used in the manufacture of
aircraft and spacecraft.
Automotive Industry
Alloys are used extensively in the automotive industry to make parts that
are strong, lightweight, and corrosion-resistant. Common alloys used in this
industry include aluminum alloys, steel alloys, and magnesium alloys.
Construction Industry
Alloys are used in the construction industry to make building materials that
are strong, durable, and corrosion-resistant. Examples of alloys used in
construction include steel alloys, aluminum alloys, and copper alloys.
Electronics Industry
Alloys are used in the electronics industry to make components that have
desirable electrical properties. For example, alloys of copper and nickel
are used to make resistance wires for heating elements, while aluminum
alloys are used to make electrical conductors.
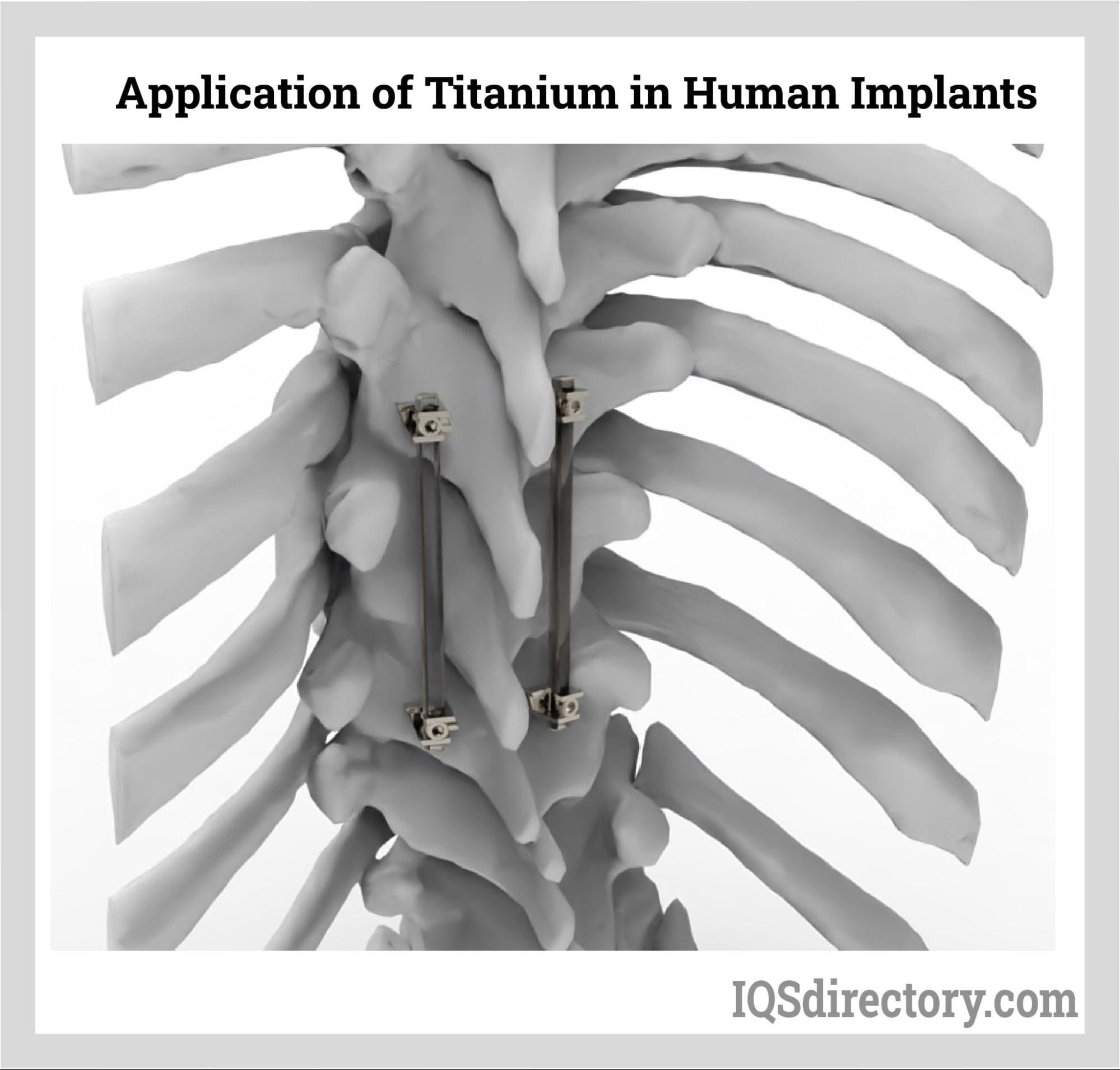
Medical Industry
Alloys are used in the medical industry to make surgical instruments,
implants, and other medical devices. Titanium alloys, stainless steel
alloys, and cobalt-chromium alloys are commonly used in medical applications
due to their biocompatibility, strength, and corrosion resistance.
Jewelry Industry
Alloys are commonly used in the jewelry industry to make precious metals
more durable and less expensive. For example, 14-karat gold is an alloy made
by combining pure gold with other metals such as copper, silver, or nickel.
Food Industry
Alloys are used in the food industry to make cooking equipment that is
resistant to corrosion and oxidation. Stainless steel alloys are commonly
used in this industry to make pots, pans, and other cooking utensils.
Choosing the Right Alloy Manufacturer
To ensure you have the most beneficial outcome when purchasing alloys from
an alloy manufacturer, it is important to compare several companies using
our directory of alloy manufacturers. Each alloy manufacturer has a business
profile page highlighting their areas of experience and capabilities, along
with a contact form to communicate with the manufacturer for more
information or request a quote. Review each alloy business website using our
proprietary website previewer to quickly learn what each company specializes
in. Then, use our simple RFQ form to contact multiple alloy companies with
the same form.